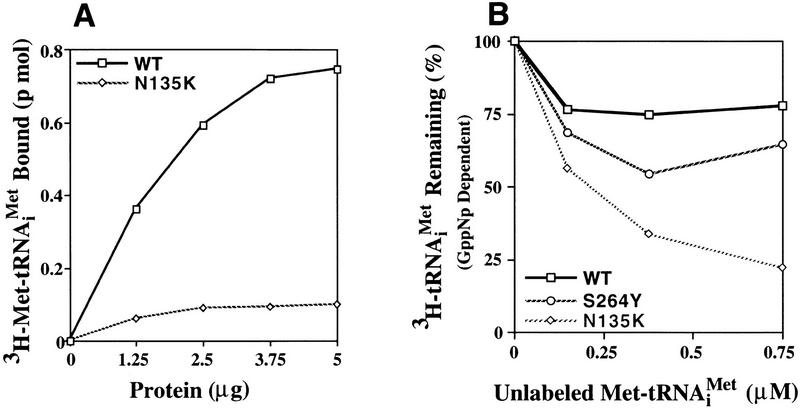
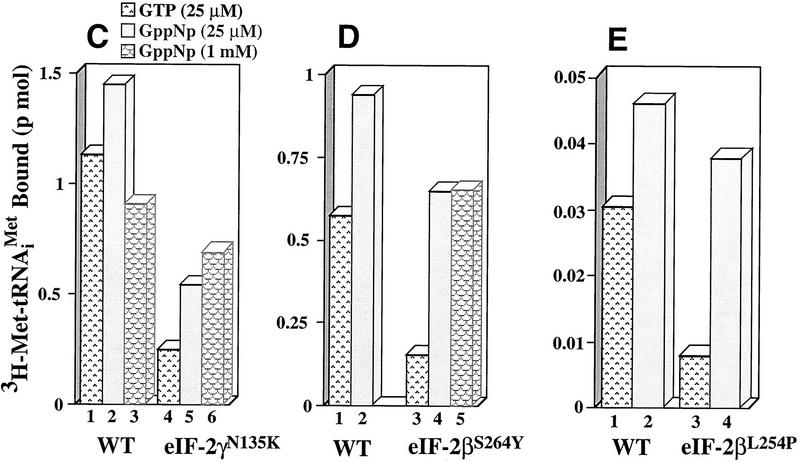
Figure 3.
Ternary complex formation by purified wild-type and mutant eIF-2 complexes. (A) Purified wild-type eIF-2 (□) and mutant eIF-2γN135K (⋄) were each assayed for the ability to promote GTP-dependent binding to the [3H] methionine charged initiator-tRNA [3H]Met–tRNAiMet, 60,000 cpm/pmole, 0.075 μm) as a function of protein concentration (μg). Identical reactions without GTP were performed as a control for nonspecific GTP-independent binding activity. The number of picomoles of [3H]Met–tRNAiMet bound in the absence of GTP was subtracted from the number of pimoles of [3H]Met–tRNAiMet] bound in the presence of GTP to determine the GTP-dependent-binding activity, for each respective eIF-2 complex. (B) Wild-type eIF-2 (□), the mutant eIF-2γN135K complex (⋄), and the mutant eIF-2βS264Y complex (○) were each assayed for their ability to dissociate from the [3H]Met–tRNAiMet in the presence of the nonhydrolyzable GTP analog, GppNp. For the wild-type eIF-2 and eIF-2βS264Y complexes, ternary complex formed after 5 min of incubation with GppNp and [3H]Met–tRNAiMet (0.075 μm) was competed with various concentrations of unlabeled, charged initiator-tRNA for an additional 5 min and the level of labeled ternary complex determined by the filter-binding assay. The same reaction conditions were used to assay the eIF-2γN135K complex with the exception that a 10-min incubation time was used in the initial step to enhance the level of labeled ternary complex. Ternary complex without addition of unlabeled charged initiator-tRNA was stable at 37°C for up to 15 min (data not shown). Identical reactions without GppNp were performed as a control for nonspecific binding of the labeled tRNA and subtracted from the respective assays as background. The amount of eIF-2 preparation in each reaction was adjusted to compensate for similar initial levels of ternary complex formation in the presence of GppNp. Total protein in each reaction was 1.25 μg for the wild-type eIF-2 and 5 μg for each of the mutant complexes. (C) The eIF-2γN135K complex (5 μg of total protein) was assayed for its ability to bind [3H]Met–tRNAiMet (60,000 cpm/pmole) in the presence of GppNp as compared with to the wild-type eIF-2 (5 μg of total protein). (Lanes 1–3) The amount of ternary complex formed by wild-type eIF-2 in the presence of GTP (25 μm), and 25 μm, and 1 mm GppNp, respectively, in a 5-min reaction. (Lanes 4–6) The amount of ternary complex formed by mutant eIF-2γN135K complex in the presence of GTP (25 μm); and 25 μm, and 1 mm GppNp, respectively, in a 10-min reaction. The 10-min time point used for the mutant complex analysis was to maximize the amount of initiator-tRNA binding. However, only a 10% increase in binding is observed by using a 10-min incubation vs. a 5-min incubation period. (D) Same as C except using purified mutant eIF-2βS264Y complex (5 μg of total protein) compared with wild-type eIF-2 complex (2.5 μg of total protein). The different levels of total protein added to each reaction adjusts for the lower yields of eIF-2 in the former preparation as determined by Western blot analysis using antibodies directed against the α subunit of eIF-2. (Lanes 1,2) The amount of ternary complex formed by wild-type eIF-2 in the presence of GTP (25 μm); and 25 μm GppNp, respectively, in a 5-min reaction. (Lanes 3–5) The amount of ternary complex formed by mutant eIF-2βS264Y complex in the presence of GTP (25 μm), and 25 μm, and 1 mm GppNp, respectively, in a 5-min reaction. (E) Same as C except using purified mutant eIF-2βL254P complex (5 μg of total protein) compared with wild-type eIF-2 complex (0.17 μg of total protein). The different levels of total protein added to each reaction adjusts for the lower yields of eIF-2 in the former preparation as determined by Western blot analysis using antibodies directed against the α subunit of eIF-2. (Lanes 1,2) The amount of ternary complex formed by wild-type eIF-2 in the presence of GTP (25 μm), and 25 μm GppNp, respectively in a 5-min reaction. (Lanes 3,4) The amount of ternary complex formed by mutant eIF-2βL254P complex in the presence of GTP (25 μm); and 25 μm GppNp, respectively, in a 5-min reaction. The data in panels B–E represent the average of two independent experiments with a standard deviation <15%.