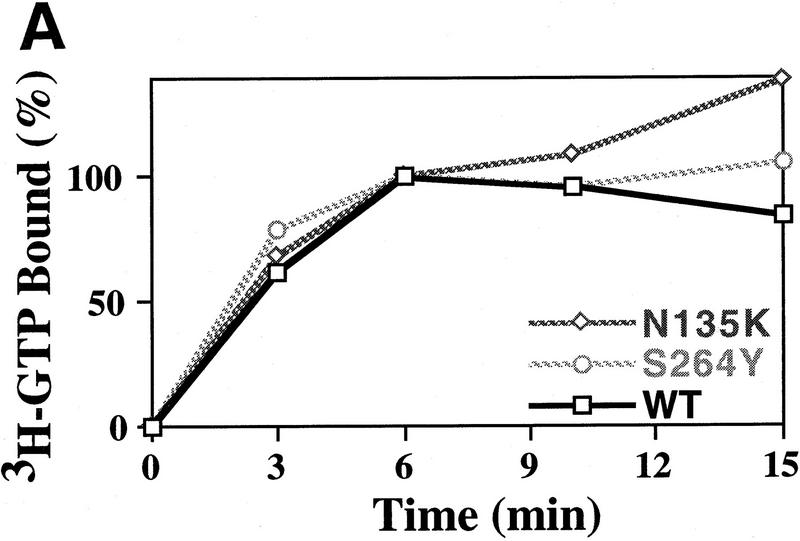
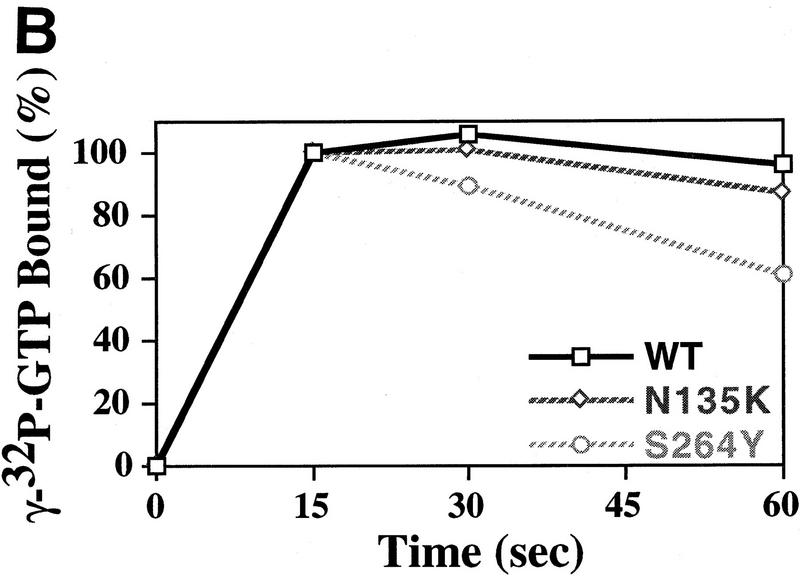
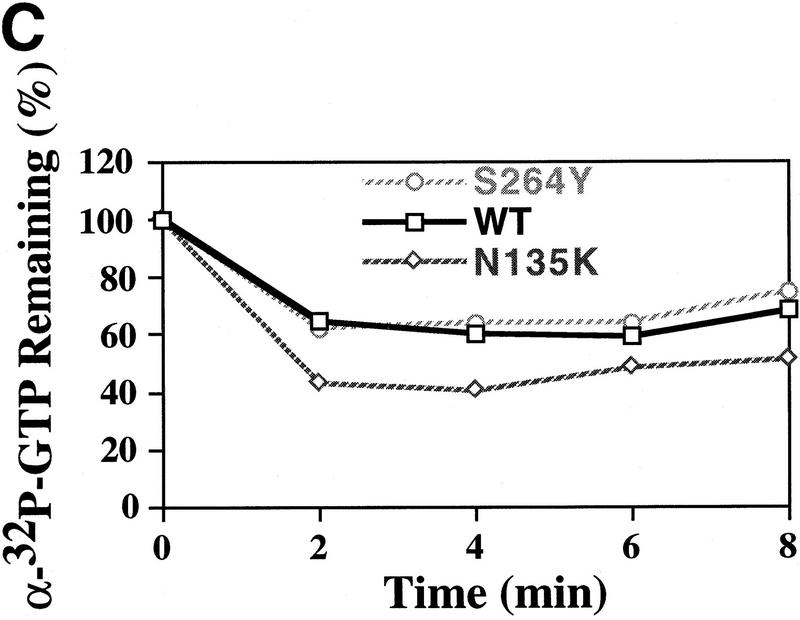
Figure 4.
GTP-binding activity of wild-type and mutant eIF-2 complexes. (A) Purified wild-type eIF-2 complex (□; 1.25 μg of total protein), mutant eIF-2γN135K complex (⋄; 1.25 μg of total protein) and mutant eIF-2βS264Y (○; 2.5 μg of total protein) were assayed for their ability to bind [3H]GTP (1 μm final concentration) using a filter-binding assay. The different levels of total protein added to each reaction adjusts for the lower yields of eIF-2 in the latter preparation as determined by Western blot analysis using antibodies directed against the α subunit of eIF-2. The number of picomoles of [3H]GTP bound in the absence of protein was subtracted from the number of picomoles of [3H]GTP bound in the presence of protein to determine the binding activity. The amount of [3H]GTP bound at the 6-min timepoint was arbitrarily chosen as the 100% level for comparative purposes. (B) Same as A except using [γ-32P]GTP. For this reaction the specific activity of [γ-32P]GTP was adjusted with [3H]GTP, using a [3H]GTP:[γ-32P]GTP ratio of 1000:1 (10 μm final concentration), as [3H]GTP is purer than unlabeled GTP (see Materials and Methods). The protein–[γ-32P]GTP complex was quantitated using a scintillation counter but without scintillation fluid to avoid interference with [3H]GTP counts. The data represent the average of two independent experiments with a standard deviation <7%. (C) Purified wild-type eIF-2 complex (□; 1.25 μg of total protein), mutant eIF-2γN135K complex (⋄; 1.25 μg of total protein), and mutant eIF-2βS264Y (○; 2.5 μg of total protein) were assayed for their ability to dissociate from [α-32P]GTP (1 μm final concentration) using a filter-binding assay. The different levels of total protein added to each reaction adjusts for the lower yields of eIF-2 in the latter preparations as determined by Western blot analysis using antibodies directed against the α subunit of eIF-2. For this reaction the specific activity of [α-32P]GTP was adjusted with [3H]GTP, using a [3H]GTP:[α-32P]GTP ratio of 100:1 (1 μm final concentration), as [3H]GTP is purer than unlabeled GTP (see Material and Methods). The protein–[α-32P]GTP complex was quantitated using a scintillation counter but without scintillation fluid to avoid interference with [3H]GTP counts.