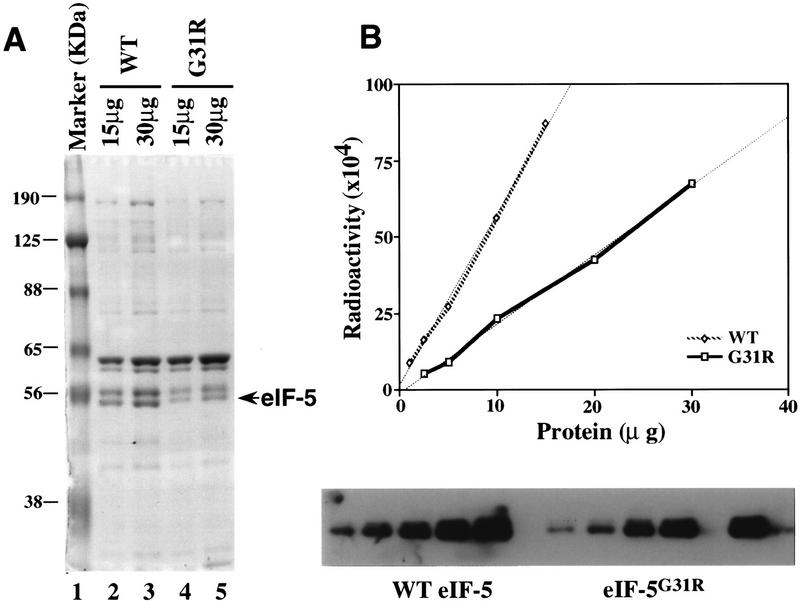
Figure 5.
Purification and quantitation of wild-type and mutant eIF-5. (A) Coomassie blue staining of purified wild-type and mutant eIF-5 protein. Protein was resolved by 10% SDS-acrylamide gel electrophoresis. (Lane 1) Molecular mass markers, the positions of which are noted as kD; (lanes 2,3) 15 and 30 μg, respectively, of concentrated wild-type eIF-5 from the Ni affinity column; (lanes 4,5) 15 and 30 μg, respectively, of concentrated mutant eIF-5G31R from the Ni affinity column. (B) Quantitation of the level of wild-type eIF-5 and mutant eIF-5G31R by Western blot analysis. Five different amounts of total protein in both the wild-type (⋄) and mutant eIF-5 (□) purified preparations were resolved on a 10% SDS-acrylamide gel and transferred to a nitrocellulose membrane and detected by Western blot analysis (bottom) using antiserum directed against eIF-5 protein (1:25,000). 125I-labeled protein A (Amersham, 30 mCi/mg) was used as the secondary probe (1:4000). The membrane was also exposed to a PhosphorImager screen (Molecular Dynamics) to determine levels of radioactivity. Data were plotted using a linear-regression program (CA-Cricket Graph III; Computer Associates) in a Macintosh computer (top). The ratio of the two slopes (WT:G31R = 2.67:1) was used as the difference in eIF-5 protein levels in the two preparations.