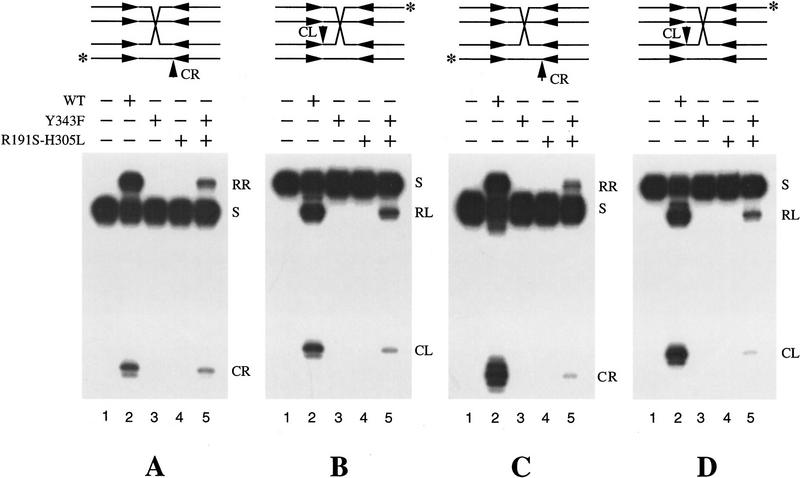
Figure 2.
Resolution of Holliday junctions by a pair of Flp step–arrest mutants. The synthetic Holliday junctions used in the assay are schematically represented at the top. The Flp-binding elements have the following sequence: 5′-GAAGTTCC TATAC-3′/3′-CTTCAAGGATATG-5′. In one (A,B), the junction was freely mobile through the spacer (5′-TTTCAGAA-3′/3′-AAAGTCTT-5′) on both DNA partners. In the other (C,D), the junction point was immobilized by flanking it with two positions of heterology on either side. This substrate mimicked a crossover between two spacers with the following sequences: 5′-TTTCAGAA-3′/3′-AAAGTCTT-5′ and 5′-TTCACAAA-3′/3′-AAGTGTTT-5′. The position of crossover was between nucleotide positions 4 and 5 of the spacer. The position of the 3′ end label in each experimental set (A–D) is indicated by the asterisk (*). The reactions were analyzed by electrophoresis in 10% denaturing polyacrylamide gels. The bands labeled RL and RR refer to resolution products from cleavage/exchange at the left and right ends, respectively. (CL and CR) Strand cleavage products from the left and right ends, respectively. (S) The labeled substrate band. Lanes 1–5 represent, respectively, reactions containing no added protein, wild-type Flp, Flp(Y343F), Flp(R191S, H305L), and an approximately equimolar mixture of the two Flp variants. Densitometric scans estimated the ratio of the intensities of the RR (or RL) bands in lanes 5 and 2 of each panel (A–D) to be ∼10%–15%.