Figure 2.
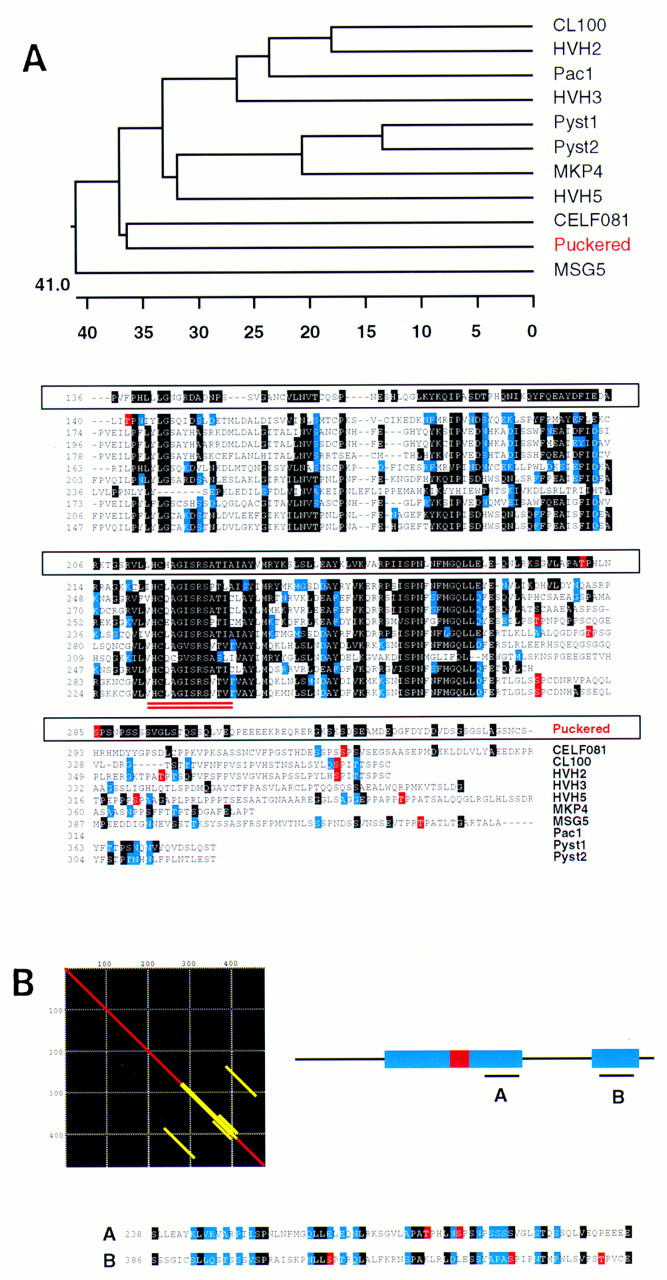
Homology of Puc to VH-1 family phosphatases. (A) Sequence alignment (ClustalV) of Puc and other VH-1 family phosphatases [Drosophila Puc; human CL100 (Keyse and Emslie 1992); human Pac1 (Rohan et al. 1993); human HVH-2 (Guan and Butch 1995); human HVH-3 (Ishibashi et al. 1994; Kwak and Dixon 1995); human HVH-5 (Martell et al. 1995); human Pyst1 (Groom et al. 1996); human Pyst2 (Groom et al. 1996); human MKP-4 (Muda et al. 1997); C. elegans CEL-F08B1 (Wilson et al. 1994) and Saccharomyces cerevisiae MSG5 (Doi et al. 1994)]. Identical residues are in black, conservative changes in blue. CEL-F08B1, Pyst1 and homologs, Pyst2, and HVH-5 gave the highest homology scores to Puc in BLAST/BEAUTY searches (BCM Launcher). The other enzymes complete the whole series of distinct human dual phosphatases isolated so far. Yeast MSG-5, which share some characteristics with Puc, is also included. Phylogenetic trees (DNAstar program) point to the C. elegans CEL-F08B1 as the closest relative of Puc in the databases. CEL-F08B1 has been identified recently in the C. elegans Genome Project, but its function is unknown. Residues in the alignment highlighted in red represent putative MAPK phosphorylation sites. Interestingly, they seem to cluster for almost every protein in a low homology region at the carboxy-terminal end of the catalytic domain (double underlined), which suggest a possible functional homology. (B) Matrix alignment of Puc with itself shows the existence of an internal repeat in the protein. These domains correspond to the putative MAPK phosphorylated region and a further sequence close to the carboxy-terminal end of the molecule. Again, in the second repeat, several tentative phosphorylation sites can be identified.
