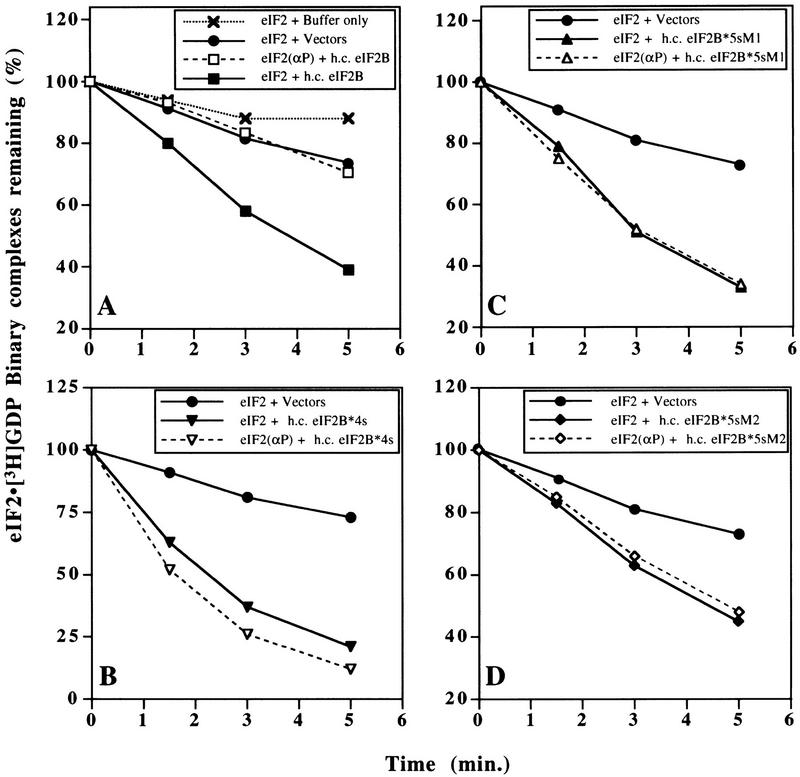
Figure 1.
Guanine–nucleotide exchange catalyzed by wild-type and mutant eIF2B, and its inhibition by phosphorylation of eIF2. eIF2 ⋅ [3H]GDP binary complexes were preformed, with or without prior phosphorylation of eIF2 by HCR, and challenged with nonradiolabeled GDP (see Materials and Methods). (A–D) Level of eIF2 ⋅ 3H]GDP (filled symbols connected with solid lines) and eIF2(αP) ⋅ [3H]GDP (open symbols connected with broken lines) binary complexes remaining with time, following incubation with cell extracts (150 μg) from yeast strains bearing high-copy plasmids encoding the form of eIF2B indicated in each inset: (A) Wild-type five-subunit eIF2B (h.c. eIF2B); (B) eIF2B*4s, regulatory mutant eIF2B containing the four essential eIF2B subunits, but lacking GCN3; (C) eIF2B*5s-M1, eIF2B containing five eIF2B subunits with a regulatory mutation in GCD7 (GCD7-S119P); and (D) eIF2B*5s-M2, eIF2B containing five eIF2B subunits with a regulatory mutation in GCD7 (GCD7-I118T,D178Y). Control reactions with cell extracts from yeast strains containing empty vectors (filled circles linked with solid lines) are shown in A–D, and a reaction with extract buffer substituted for yeast cell extract (crosses linked by dotted lines) is shown in A.