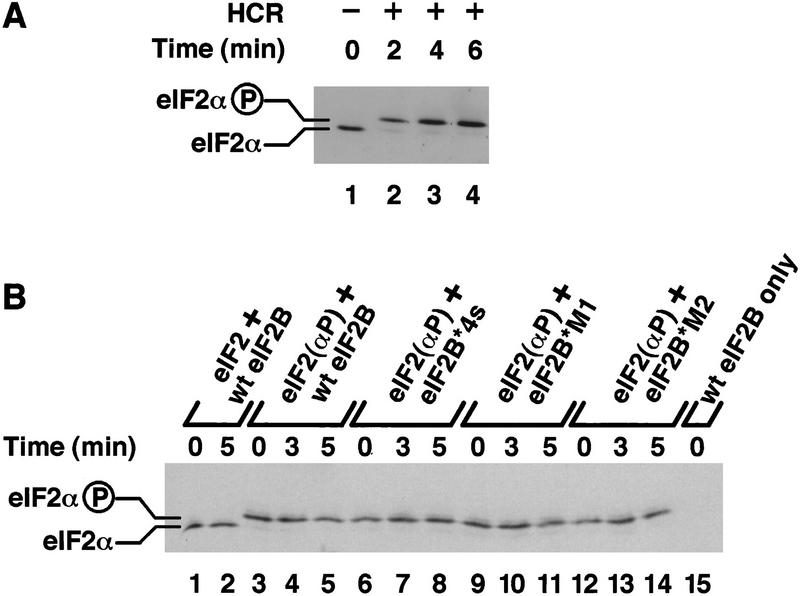
Figure 2.
Phosphorylation of purified eIF2 in vitro. (A) Purified eIF2 was phosphorylated for the indicated times (lanes 2–4), after which samples were resolved by IEF PAGE and eIF2α detected by Western blotting. (Lane 1) Unphosphorylated purified eIF2. (B) Guanine–nucleotide exchange reactions were performed exactly as described in Fig. 1, except that unlabeled GDP was used throughout. Samples were taken at the indicated times following the addition of cell extracts (150 μg) from yeast strains bearing high-copy plasmids encoding the indicated form of eIF2B (as in Fig. 1). eIF2α was resolved and detected as in A. (Lanes 1,2) Samples from reactions with unphosphorylated eIF2 ⋅ GDP binary complexes; (lanes 3–14) binary complexes prephosphorylated with HCR kinase; (lane 15) buffer and wild-type eIF2B extract only with no added eIF2 ⋅ GDP binary complexes. The positions of phosphorylated and unphosphorylated eIF2α are indicated.