Abstract
Lake St. Lucia, iSimangaliso Wetland Park, South Africa, is the largest estuarine lake in Africa. Extensive use and manipulation of the rivers flowing into it have reduced freshwater inflow, and the lake has also been subject to a drought of 10 years. For much of this time, the estuary has been closed to the Indian Ocean, and salinities have progressively risen throughout the system, impacting the biotic components of the ecosystem, reducing zooplankton and macrobenthic biomass and diversity in particular. In June 2009, a bloom of a red/orange planktonic microorganism was noted throughout the upper reaches of Lake St. Lucia. The bloom persisted for at least 18 months, making it the longest such bloom on record. The causative organism was characterized by light and electron microscopy and by 16S rRNA sequencing and was shown to be a large, unicellular cyanobacterium most strongly associated with the genus Cyanothece. The extent and persistence of the bloom appears to be unique to Lake St. Lucia, and it is suggested that the organism's resistance to high temperatures, to intense insolation, and to hypersalinity as well as the absence of grazing pressure by salinity-sensitive zooplankton all contributed to its persistence as a bloom organism until a freshwater influx, due to exceptionally heavy summer rains in 2011, reduced the salinity for a sufficient length of time to produce a crash in the cyanobacterium population as a complex, low-salinity biota redeveloped.
INTRODUCTION
Persistent phytoplankton blooms in estuaries and coastal hypersaline waters have been reported on a few occasions (7, 13, 34, 35, 41, 49), but the dynamics of their development and duration are not always clear (5, 21). Cyanobacterial blooms in particular are becoming more frequent worldwide, and while usually associated with eutrophication (5, 41), they have also been linked to climatic change, including its effects on salination (36).
In June 2009, a widespread bloom of an orange-pigmented planktonic organism appeared in the upper reaches of the Lake St. Lucia estuary, South Africa, in conjunction with the development of extreme hypersaline conditions in the area. Lake St. Lucia forms part of the iSimangaliso Wetland Park, a World Heritage Site, and is the largest estuarine lake in Africa (19, 20). It has been subject to at least a century of indirect anthropogenic alteration, which has dramatically reduced the freshwater inflow to it. Extensive areas of wetlands that formerly supplied the estuary have been drained for agricultural use, and water diversion from the rivers feeding into the estuary has significantly reduced freshwater inputs (29, 52, 58). In addition, the structure of the mouth is managed to prevent the largest freshwater inflow, the Mfolozi River, from breaching fully into the estuary, ostensibly to prevent siltation, but this has resulted in prolonged closure of the mouth (58).
Climatically, the area is subject to alternating drought and wet conditions varying over decadal scales. The current 10-year-long drought is one of the longest on record, however, and has produced profound changes in the ecological conditions of the lake (4, 9, 20, 37, 39, 40, 52). Since 1992 the mouth has opened to the Indian Ocean on only two occasions: briefly in January 2004 and then for a 6-month period in 2007, when waves generated by Cyclone Gamede, combined with high equinoctial spring tides, breached the berm from the ocean side (9). This infrequent opening of the estuary mouth, coupled with very low freshwater inflow, has resulted in low water levels throughout the system and progressively rising salinities, particularly in the shallow North Lake and False Bay, where high insolation rates, shallow water levels, and high temperatures result in rapid evaporation (58). As a result, hypersalinity, at times in excess of 200 (on the practical salinity scale) has become the norm for much of the northern reaches, and a reverse salinity gradient has been established, with the lowest salinities near the mouth and hypersaline conditions to the north (20).
Under normal conditions, the whole St. Lucia system supports a diverse biotic community (20, 39, 40). However, as a result of rising salinity, many species have been eliminated from large areas of the lake, and elsewhere only the most resistant eukaryotes appear to survive (9, 37, 39, 40). Results also suggest that under hypersaline conditions, trophic systems begin to be truncated, as grazers are particularly susceptible to rising salinity, and that microalgal biomass, both benthic and planktonic, initially rises as a result (37). Above 140 on the salinity scale, however, only prokaryotes appear able to survive, and eukaryotes are eliminated from both the plankton and benthos (9).
In July 2009, a major fish kill, attributed to exceptionally cold weather, occurred throughout the St. Lucia system, including False Bay and the North Lake. At the same time, routine sampling showed that salinities had reached levels of >150 in the northern reaches and that zooplankton stocks had been almost entirely eliminated, while megafauna, such as waterbirds, crocodiles, and hippopotami, disappeared from virtually the entire region as they migrated toward the lower salinities nearer the mouth. At the same time, a widespread bloom of an orange-pigmented planktonic organism was observed throughout the North Lake and False Bay. Provisional diagnoses suggested that this bloom might be due to proliferation of a protist and algal genera, such as Chlamydamonas, Dunaliella, or a dinoflagellate, seemed likely candidates, since Grindley and Heydorn (23), for example, had reported a 2-month-long bloom of Noctiluca scintillans in the False Bay region during the winter of 1969, which was also a period of drought, hypersalinity, and an associated (although more minor) fish kill.
The 2009 bloom persisted for nearly 18 months and changed very little in appearance. When episodic heavy rains occurred, the bloom would become somewhat diluted with the added water, but it reappeared once evaporation again reduced the water volume. Finally, in January 2011, very heavy rains resulted in flooding of the entire lake system, including the northern reaches, and led to the apparent final dissolution of the bloom. The current work was initiated to provide a definitive identification of the bloom organism and preliminary information on the ecological drivers which had caused the bloom to occur and which were responsible for its lengthy persistence.
MATERIALS AND METHODS
Sample site.
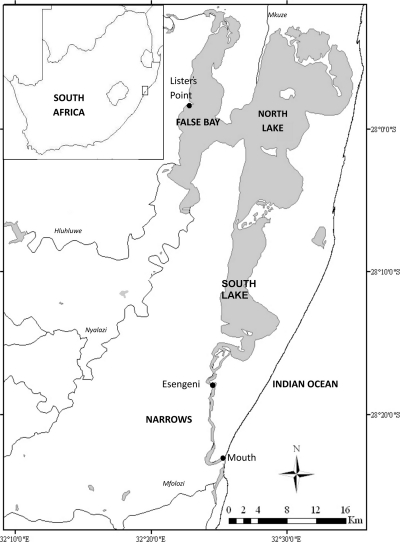
Lake St. Lucia is located on the subtropical east coast of South Africa, in a summer rainfall area where yearly rainfall totals are normally in excess of 700 mm (29, 58). The estuary consists of four physically distinct regions (Fig. 1). From the mouth, a narrow riverine channel (The Narrows) leads to the South Lake, which is tidally influenced under open-mouth conditions. This is narrowly connected across a shallow bottom to two large northern embayments (False Bay and North Lake), which are themselves shallow (<2-m depth) and only minimally tidally influenced (52, 58).
Fig. 1.
St. Lucia Estuary, iSimangaliso Wetland Park, KwaZulu-Natal, South Africa, showing both sites (Lister's Point and Esengeni) for which physico-chemical data are presented. Adapted from reference 9 with permission of the publisher.
The sample site was located in the False Bay region at Lister's Point (Fig. 1). The sample site is typical of the northern reaches, with shallow water that reaches high daytime temperatures and which is subject to seiching (wide-scale movement due to wind action). A comparison site at Esengeni in The Narrows (Fig. 1) was used to compare physico-chemical parameters.
Physico-chemical variables.
Depth, temperature (°C), pH, dissolved oxygen (mg liter−1) and salinity (on the practical salinity scale) were measured by using a YSI 6920 water quality logger fit with the appropriate probes.
Water sample collection.
Samples of 450 ml were taken in acid-washed, sterile, 1.0-liter Schott bottles. These were transported to the laboratory and maintained with loose caps in front of a bank of warm fluorescent lighting (140 μmol m−2 s−1 at the bottle surface) under a 14-h light, 10-h dark regimen at room temperature at a 30-cm distance.
Chl a determinations.
Known volumes of water were filtered onto Whatman GF/F filters under gentle vacuum and stored frozen. Chlorophyll a (Chl a) was extracted by immersing filters in cold (<5°C) 90% acetone for 24 h. The Chl a concentration was determined using a Turner Designs 10-AU fluorometer with a narrow-band, nonacidification system (57).
Cell counts.
Cell counts of the bloom organism were determined with a hemocytometer, following the methods of Guillard and Sieracki (24). Historical counts were determined from Lugol's iodine-fixed samples taken prior to February 2010. After that date, cell counts were determined from live samples within a day of collection.
Culture and enrichment responses.
Employing nutrient amounts recommended for F/2 marine algal culture medium (2), duplicate 450-ml samples of lake water were fertilized with 8.83 × 10−4 M (final concentration [f.c.]) NaNO3 and 3.63 × 10−5 M NaH2PO4·H2O (f.c.). Duplicate controls were left unfertilized. Chl a content was determined each week for a period of 5 weeks using methods described above.
Attempts were made to purify the organism by dilution plating and streaking onto SN medium (2) solidified with 1.0% (f.c.) Noble agar, but even where apparently isolated colonies arose, these never proved to be axenic, and the organism has proved recalcitrant to all attempts to establish pure culture using published methods (55).
Light microscopy.
Sample aliquots were examined on glass slides with a Nikon Eclipse 80i microscope, using phase contrast and Nomarski differential interference contrast (DIFC). To examine capsule and slime structures, cells were stained using the Anthony direct stain modification of the Hiss method (3), in which dried smears of cell suspensions were stained for 2 min with 1% (wt/vol) aqueous crystal violet and destained by rinsing with 20% (wt/vol) CuSO4·7H2O, followed by brief rinsing in distilled water to reduce salt crystal formation. Capsules were also demonstrated with a negative stain modification of the Anthony method, in which cell suspensions were mixed with skim milk (0.1% [vol/vol]) to serve as a background. The Duguid negative stain method (17) could not be used, because nigrosin and India ink both precipitated and clumped at high salinities and failed to show capsules adequately.
Epifluorescence microscopy.
Five-milliliter sample aliquots were stained with 4′,6-diaimidino-2-phenylindole (DAPI; 5 × 10−5 mg ml−1 f.c.), and subsamples were mounted on glass slides. Examination was with a Nikon Eclipse 80i, utilizing Nikon filter sets UV-2a (excitation, 330 to 380 nm; barrier filter, 420 nm) for DAPI visualization and the FITC (G2A) filter set (excitation, 510 to 560 nm; barrier filter, 590 nm) for visualization of Chl a autofluorescence.
For photomicrography we used Nikon imaging software (NIS-D) for digital image capture using a Nikon digital sight DS-Fli digital camera. Where necessary, pictures were cropped and enhanced for color and contrast, etc., using the GNU image manipulation program (GIMP-2; http://www.gimp.org; last accessed 6/23/2011).
Transmission electron microscopy (TEM).
Fresh samples were fixed in 2.5% glutaraldehyde (f.c.) before proceeding through preparation solutions. After each solution change, the samples were lightly centrifuged to form a pellet. The supernatant was then carefully removed before being replaced by the next solution in the process.
After several rinses with 0.1 M phosphate buffer, the material was postfixed in 1% osmium tetroxide solution for 1 h, washed several times with phosphate buffer, and dehydrated in a graded acetone series. The samples were infiltrated with 50:50 acetone-epoxy resin (48) overnight and further infiltrated with 100% epoxy resin for 24 h. Samples were placed in resin-filled Beem capsules and polymerized at 70°C for 8 h.
Sectioning was carried out using a Reichert Ultracut E microtome. Ultrastructural analysis was performed using ultrathin sections (60 to 100 nm), which were poststained with saturated uranyl acetate solution (10 min) followed by lead citrate (44) for 10 min. Sections were viewed with a Jeol 1010 transmission electron microscope, and images were captured using a Megaview 3 Soft imaging system (SIS).
16S rRNA amplification and sequencing.
To reduce the proportion of DNA from other organisms in the bulk DNA extraction, a centrifugation method was used to amplify the relative proportion of large cyanobacterial cells before submission for 16S rRNA sequencing. After centrifugation for 30 min at 5,000 × g, the cells under consideration failed to form a firm pellet, but other organisms, such as eubacteria and smaller cyanobacteria, did, as determined by phase-contrast microscopic examination of centrifugal pellets. As a result, repeated centrifugation and decantation of supernatant containing cells of interest led to the progressive, albeit crude amplification of large cyanobacteria relative to other organisms.
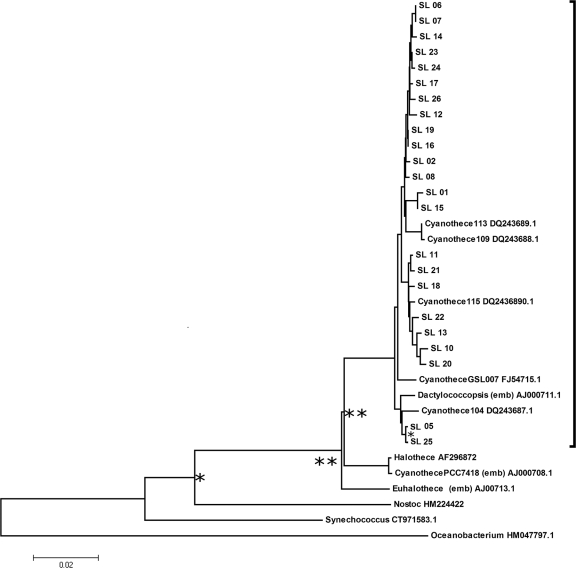
Samples of concentrated cells were sent to the Inqaba Laboratories (Inqaba Biotech, Hatfield 0028, Pretoria, South Africa) for DNA extraction, 16S rRNA gene amplification by PCR, and sequencing of 26 randomly picked clones using standard methods. Amplification and sequencing in both directions were performed using universal primers pJET-F (5′-CGACTCACTATAGGGAGAGCGGC), pJET-r (5′-AAGAACATCGATTTTCCATGGCAG), 27F (5′-AGAGTTTGATCMTGGCTCAG), and 1492r (5′-TACGGYTACCTTGTTACGACTT). Contigs for each of 26 randomly selected clones were compiled and joined, and sequences for all selected clones were aligned using the CLC Genomics Workbench 4.0.2 program (CLC-Bio, Aarhus, Denmark). The alignment was edited to attempt to match all 26 isolated clone sequences, a total of 1,400 bases, or 91% of the complete 16S rRNA gene. Three sequences were not used because the forward and reverse contigs could not be joined, as the complete sequences were excessively short. All 23 sequences used in subsequent phylogenetic analyses were designated environmental clone isolates Saint Lucia (SL) 01 to 26 and have been submitted to the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/) with designations detailed below. An initial Basic Local Alignment Search Tool (BLAST) search was done using the consensus sequence to determine approximate phylogenetic relationships, and each individual sequence was also subjected to BLAST analysis. Sequence information for organisms associated on the BLAST searches was extracted from the GenBank database for determination of phylogenetic relationships (see Fig. 7, below). The marine eubacterium Oceanobacterium iheyi was used as an outgroup, and the complete alignment of 35 sequences was again compiled using the CLC Genomics workbench.
Fig. 7.
Phylogenetic relationships of 35 taxa. Phylogenetic relationships were inferred using the neighbor-joining method (46) with Oceanobacterium iheyi (a marine eubacterium) as an outgroup. The bootstrap consensus tree inferred from 1,000 replicates was taken to represent the relationships of the taxa analyzed. Bootstrap values of >90% are marked by an asterisk. Values of >98% are marked with two asterisks. The tree is drawn to scale, with branch lengths in the same units as those of the phylogenetic distances used to infer the tree. Distances were computed using the maximum composite likelihood method (51) and are in the units of the number of base substitutions per site. Codon positions included were the first, second, third, and noncoding. All positions containing gaps and missing data were eliminated from the data set (complete deletion option). There were a total of 1,307 positions in the final data set. Analyses were conducted in MEGA4 (50). A strongly supported clade (bootstrap value, 100%), including all 23 St. Lucia (SL) isolates, together with 5 Cyanothece species and Dactylococcopsis salina identified in the NCBI (GenBank) or European Molecular Biology Laboratory (EMBL) database, is enclosed by a bracket on the right.
These sequence data were analyzed for phylogenetic relatedness by using a neighbor-joining method (46, 50) of Mega-4 (51) with a bootstrap consensus tree inferred from 1,000 replicates. Phylogenetic distances were computed using the maximum composite likelihood method, where the unit of distance is the number of base substitutions per site.
Nucleotide sequence accession numbers.
All sequences used for phylogenetic analysis were deposited in the GenBank database (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov) in January 2011 and assigned accession numbers HQ914216 to HQ14239.
RESULTS
Water chemistry.
At our initial sampling (19 February 2010), the water at the sample site was distinctly pigmented with a bright orange/red color. The water was shallow (maximum depth, 50 cm) and recorded mid-day water temperatures were high (55°C), with a pH of 8.1. The oxygen content was 8.17 mg liter−1 (100% saturation). Large banks of brown, stable foam were present on the beaches, and foam could be seen forming on the water surface as increasing wind speed caused water movement across the shallow embayment. The photosynthetically available radiation (PAR) at the surface at the time of sampling was 530.4 μmol m−2 s−1. Because of the shallowness of the water, it was not possible to calculate the Kd, but Perissinotto et al. (37) have recorded even higher PAR values (>2,000 μmol m−2 s−1) in the system, although the Kd may also be extremely high (0.9 to 36.1 m−1), depending on depth, turbidity, and phytoplankton density.
Salinities recorded for the past 5.5 years at this site and one other site in The Narrows (Esengeni) are presented in Fig. 2. These show that the water in False Bay is frequently hypersaline compared both to seawater and to the lower reaches of the lake system. There is normally a seasonal drop in salinity as the effects of the summer rainy season become apparent through increased freshwater inflow, but despite this, hypersaline conditions predominate in the northern reaches. The lowest salinity for the period of record was 18.3, during summer rains when the mouth was open (2007). At our initial sampling for the bloom organism in February 2010, a salinity of 220 was recorded.
Fig. 2.
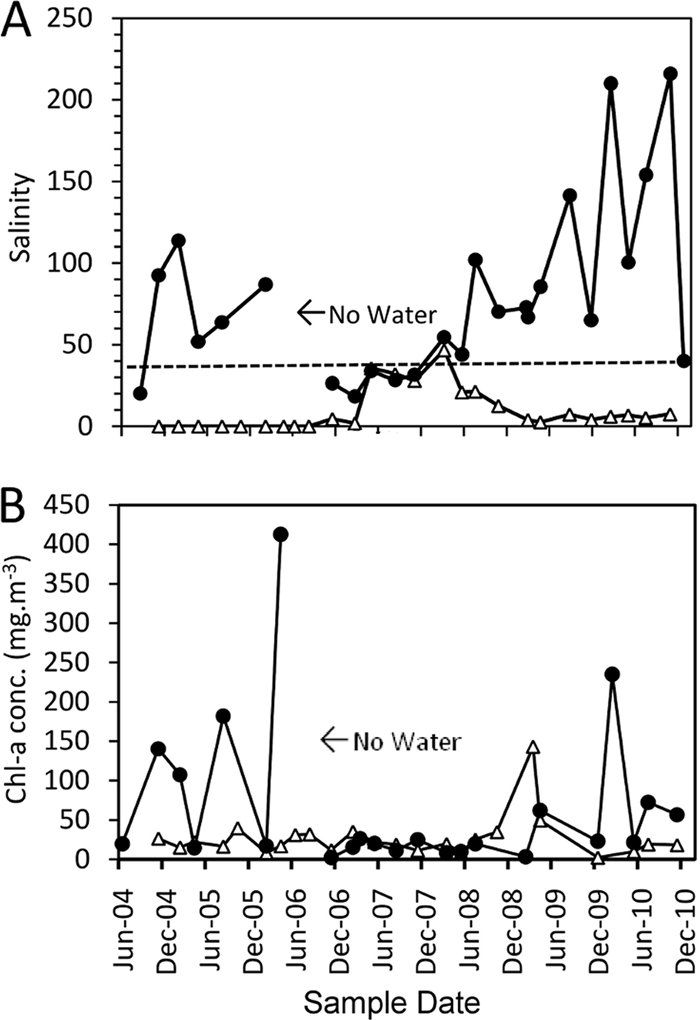
Salinity on the practical salinity scale (A) and Chl a concentrations (in mg m−3) at Lister's Point (●) and Esengeni (▵) for the period June 2004 to January 2011. For a period of at least 2 months in 2006 (marked on the diagram), there was no water at the False Bay site (55). In panel A, the dotted line represents the salinity of seawater (34.5).
Cell counts.
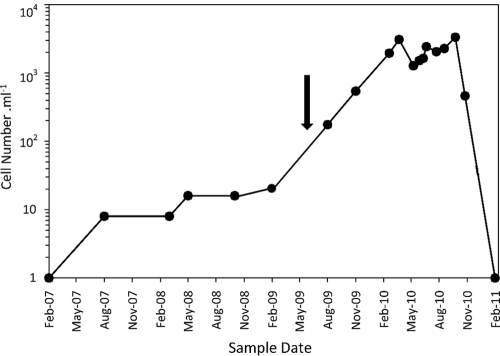
In historical samples, cells similar to the organism of interest were first noted in May 2007 (8 to 10 cells ml−1) and were present in very low numbers throughout the austral autumn and winter of 2007 (Fig. 3). A slight increase in numbers commenced in the spring and summer of 2008 (10 to 20 cells ml−1), but between February 2009 and August 2009 the organism bloomed, reaching numbers of >500 cells ml−1 in July 2009, when the bloom was first noted, and >1,000 cells ml−1 by January 2010. The cell counts throughout 2010 remained high (mean ± standard deviation, 2,031 ± 586 cells ml−1) until November, when they began to fall at the onset of summer rains. Following the water level rise after very heavy rains in January 2011 (due in part to La Niña effects that were apparent globally throughout the Southern Hemisphere), the bloom crashed and cells were then undetectable for hemocytometer counts.
Fig. 3.
Cyanothece sp. cell count (cells ml−1) determined by hemocytometer with Lugol's iodine-preserved samples (February 2007 to November 2009) and fresh samples (February 2010 to February 2011). The approximate point at which the bloom became visible is marked by an arrow.
Cell structure.
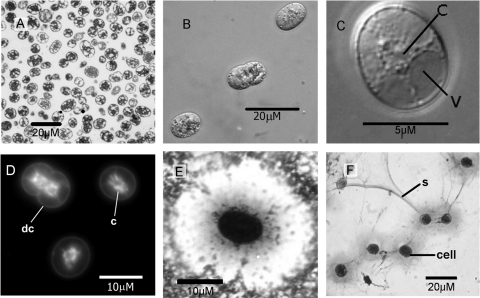
Cells were spherical to ovoid, 8 to 10 μm in length, and approximately 7 μm across, with a distinct cell wall (Fig. 4A and C). The cells were nonmotile. Ovoid cells divided by binary fission at right angles to the long axis (Fig. 4D). Spherical daughter cells may adhere for some time. There was a loosely adherent capsule (Fig. 4E) of variable thickness, and cells may also produce copious amounts of mucilaginous slime, but there was no true slime sheath (Fig. 4F). There was abundant internal membrane structure and a variety of cellular inclusions, such as phase-bright and phase-dark granules, which varied in size and frequency between different cells (Fig. 4B and C). Internally, the cytoplasm in naturally occurring cells is usually constrained to a distinct star-shaped mass by large peripheral vacuoles that extend from the cell membrane. These vacuoles were clearly visible under both light microscopy, using phase contrast (Fig. 4C), and TEM (Fig. 4A and 5). The content of the vacuoles is unknown, but in other cyanobacteria, similar vacuoles have been shown to contain lipids, glycogen, or gas (53, 56). The stellate vacuolar structure may be specific to this strain of Cyanothece: it has not been described for C. aeruginosa, the type species of the genus (26), nor for strains BH63 and BH68 (43), C. halobia (10), or C. minervae/cedrorum (28), three strains described by Porta et al. (42), nor for any others we have considered from the literature, such as the study reported by Garcia-Pichel et al. (22).
Fig. 4.
Cell morphologies of cells collected in February 2010 (salinity, >200). (A) Thin section of resin-embedded cells, visualized by low-magnification TEM. (B) Live cells viewed by Nomarski differential interference contrast with Z-stacking of several levels of focus, displaying the prominent granular nature of the cytoplasm. (C) Phase-contrast micrograph with Z-stacking of a single cell, showing typical peripheral vacuoles (v) with granular cytoplasm (c) constrained to a star-shaped mass at the center of the cell. (D) DAPI-stained cells viewed by epifluorescence microscopy, showing cytoplasm (c), distinguished by reticulate and rod-shaped, brightly fluorescent DNA. dc, dividing cell. (E) Typical capsular structure, visualized by negative staining (skim milk background) by a modification of the Anthony method (3). The central, deeply stained cell is surrounded by a lightly stained capsule against the dark background of stained milk proteins. (F) Demonstration of slime production using the Anthony method (3) for direct staining of slime and capsules. Capsules are visible around the deeply stained cells as fainter areas of staining, with prominent slime strands (s) extending between cell groups.
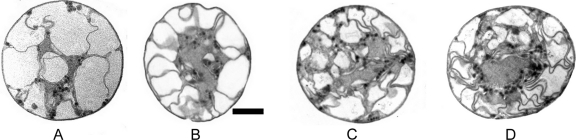
Fig. 5.
Micrographs of thin sections of cells maintained at a salinity of 200, at various times after addition of nutrient (NaNO3 or NaH2PO4). (A) Nutrient-starved cell; (B) 1 week after nutrient addition; (C) 2 weeks after nutrient addition; (D) 3 weeks after nutrient addition. Bar, 1 μM.
Nuclear staining with DAPI showed that there was no distinct nucleoid but that DNA was distributed throughout the central cytoplasm mass, forming distinct thick filaments constrained to the cytoplasm within the boundaries of the peripheral vacuoles (Fig. 4D). There were regional areas where the DNA is more brightly fluorescent than elsewhere. There was little change in DNA distribution during cell division, as revealed by DAPI staining (Fig. 4D).
Viewed under an epifluorescent wavelength of 510 to 560 nm, freshly sampled cells were autofluorescent with a variable brightness that was red, typical of chlorophyll. The autofluorescence showed filaments and plates distributed throughout the cytoplasm, in a manner strongly suggestive of the thylakoids characteristic of other unicellular, diazotrophic cyanobacteria (28, 43). They are typically arranged in a net-like fashion (Fig. 5), described as “keritomization” by Komárek and Cepak (28).
We noted that after cells had been maintained for 2 months without addition of nutrients, the culture gradually paled in color from the original red/orange to a straw yellow/orange. Cells from such cultures also lost the bright autofluorescence of freshly sampled cells, although autofluorescence was never entirely lost, and the volume of cytoplasm decreased dramatically (Fig. 5A) with a concomitant increase in vacuole volumes. Further, cells that had been fertilized developed a brilliant red autofluorescence as their pigmentation changed from red to blue-green. This could be maintained for more than 6 months even without further fertilization, and only then slowly fading to resemble environmentally collected cells.
TEM showed that cells were uniformly circular in cross-section with a thin but distinct cell wall (Fig. 5). The large vacuoles previously described were clearly visible, bounded by the cell membrane and thylakoids on the outer periphery and by a stellate arrangement of cytoplasm and thylakoids that extended inwards to the central cytoplasm itself (Fig. 4A and 5).
Effects of fertilization.
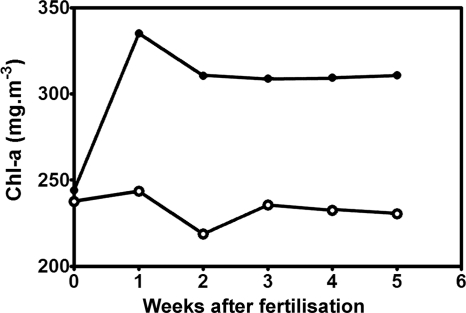
Both electron microscopy (Fig. 5A to D) and Chl a results (Fig. 6) showed that there was an almost immediate response to nutrient addition as the internal structures of the cells of interest became substantially reorganized within a week: the amount of cytoplasm increased, vacuoles began to be subdivided by production of dense and elaborate thylakoid structures (Fig. 5A to D), and cells developed a bright blue-green pigmentation. There was a 42% increase in Chl a content that was maintained for at least another month (Fig. 6). It was evident that thylakoid development continued for at least 3 weeks (Fig. 5D).
Fig. 6.
Chlorophyll a content (mg m−3) after addition of NaNo3 and NaH2PO4·H2O. ●, experimental; ○, control.
Nutrient dynamics of this organism are the subject of ongoing research, since these results reflect the addition of high levels of nutrient at very high salinity. Responses to more realistic levels of nutrient at low salinities remain to be determined.
16S rRNA sequence analysis.
BLAST searching with each St. Lucia isolate sequence against the NCBI database of bacterial 16S rRNA sequences and genomes showed similar results. The organisms were placed within the Cyanobacteria, with each being most closely associated with identified Cyanothece sequences, with some partial identification to the genus Dactylococcopsis and with a more remote relatedness to the genera Halothece and Euhalothece. Similarity cluster analysis by the neighbor-joining method (46, 50) of 26 cloned sequences suggested that a population of very closely related organisms had been sampled, albeit with some degree of polymorphism. Nonetheless, all 26 clones showed a high degree of similarity, and a preliminary consensus sequence was derived, which was itself subjected to BLAST searching. This confirmed the placement of the organism within the cyanobacteria, most closely associated with members of the genus Cyanothece and to other cyanobacteria associated with hypersaline conditions, such as in salt ponds (Dactylococcopsis sp.).
The 23 most complete sequences were then compared against selected identified sequences that BLAST searching had shown to have a range of relatedness (Fig. 7). This revealed a single major clade (bootstrap value, 100%) containing all 23 environmental clones from St. Lucia, which grouped together with various Cyanothece sequences and that of Dactylococcopsis, with a close relationship to a separate grouping of Cyanothece PCC 7418, Halothece, and Euhalothece and with extremely remote relationships to both Synechococcus and Nostoc.
DISCUSSION
We are confident that the organism responsible for this bloom is a cyanobacterium that is a member of the genus Cyanothece (Synechococcaceae, Chroococcales). The general morphology conforms to descriptions of the genus as established by Komárek (26, 27) and elaborated on by Waterbury and Rippka (56) and Komárek and Cepak (28). This morphology of the cytoplasm does, however, bear a striking resemblance to the internal organization of Dactylococcopsis salina as shown by Van Rijn and Cohen (53), and although our phylogenetic analysis certainly suggests some relatedness between the two, the overall morphology is quite distinct, since Dactylococcopsis is a sickle- or spindle-shaped cell, where the organism here preserves the spherical or ovate-ellipsoid form typical of Cyanothece. In Dactylococcopsis salina, the corresponding vacuolar structures are gas filled and provide a sensitive flotation control, which maintains cells under optimum conditions in the water column (54). The content and function of vacuoles in Cyanothece recovered from Lake St. Lucia remain to be determined.
Although cells have no true slime tube, there is a prominent capsule, and cells produce a large amount of a mucilaginous slime that remains only loosely adherent to the cells but which can be demonstrated by staining using the Anthony method (Fig. 4F). With wind or wave action, this slime contributes to the formation of large amounts of a stable foam, which is characteristic of those areas of Lake St. Lucia where the bloom is present. Similarly, prolific exopolysaccharide production has been demonstrated for several other Cyanothece species and strains and may account for a substantial proportion of cellular production (11, 15, 16). Not only may exopolymers provide a direct trophic resource (14), but also the resulting foam may have an additional important ecological function. Blown up onto the beaches, it remains as a stable layer that is continually replenished as wind action moves fresh foam onshore. If these foam banks are moved aside, a distinctly wet layer of substratum with a prominent, active microbial mat is revealed beneath them. While extremely hypersaline, the environment beneath the foam banks is moist and shielded from the intense heat and insolation characteristic of the dry, bare mudflats that comprise the uncovered beach. Bate and Smailes (4) have pointed out the importance of refugia such as these in maintaining diatom populations in particular, and we are certain that close examination would show a complex associated bacterial flora as well. We also believe that the capsule and associated slime contribute to the difficulty of establishing axenic cultures of this organism, since other bacteria and smaller cyanobacteria are very frequently observed to associate with the enveloping polysaccharides.
Members of the genus Cyanothece are commonly associated with extreme conditions similar to those in Lake St. Lucia (22). For example, C. halobia was isolated from saltworks (45) and De Philippis et al. (16) isolated the genus from a Somali saltpan and subsequently described 14 other strains from similar habitats (15), while Margheri et al. (32) described a further 11 strains isolated from geographically disparate saltworks, hypersaline ponds, tidal pools, solar lakes, and the Dead Sea.
Cells similar to those of the bloom were present in low numbers in water samples as early as June 2007, a year before the bloom began, when salinities, while high (>70), were far below the maximum observed later (>200). Routine sampling showed that a diverse planktonic and benthic trophic community was still present at these lower salinities (9). As salinities rose above 100 in 2009; however, this community was truncated. In particular, eukaryotes, such as ciliates, phytoflagellates, and the entire zooplankton community, disappeared (although a complex prokaryotic community survived, in addition to the bloom organisms). With the loss of the predatory community, the number of cyanobacterial cells began to increase. The fish kill, due to cold weather in July 2009, occurred when water levels were reduced at the end of the lengthy dry winter season in a year where rainfall had already been lowered by drought. Although hypersaline conditions were present, the salinity at first notice of the bloom was 100 or less, conditions much more conducive to rapid cell division than the later salinities of >150, and other authors have noted that nutrient requirements of phytoplankton increase at higher salinities and that even organisms tolerant of hypersalinity experience increased metabolic demands (27, 29).
We are unable to say what sparked the onset of the bloom conditions, but we speculate that a transient nutrient pulse led to the initial increase in numbers of Cyanothece cells sufficient to produce bloom conditions by late June 2009 and that the later fish kill further augmented nutrients. We have noted that even a brief exposure to high nutrient levels results in a very rapid restructuring of the cell architecture and swift upregulation of photosynthetic pigments, which then persist for many months (Fig. 5 and 6), and that even environmental, unfertilized samples can be maintained, virtually unchanged, for more than a year. Perissinotto et al. (33) have also shown that dissolved inorganic nitrate and phosphate are seldom at limiting concentrations in Lake St. Lucia and may thus be high enough for maintenance of the bloom through efficient regeneration of nutrients. Without any grazing pressure from ciliates or zooplankton, and with resistance to hypersalinity and extreme light and heat conditions, the bloom organism, once established, would survive in the absence of other competitive organisms.
Although Cyanothece has been isolated from marine and hypersaline environments frequently (11, 12, 15, 32, 43) and there is considerable interest in the diazotrophic activity of several of the members of the genus (43), there is very little information in the literature regarding its general ecology or that of any of the other large, nanoplanktonic unicellular cyanobacteria. The only similar reports are from work with Dactylococcopsis salina recovered from Solar Lake, Sinai, which undergoes seasonal population expansions and becomes spatially concentrated in the lake, forming a dense narrow band constrained by its intolerance of H2S in deeper water and high insolation and zooplankton predation in the upper layers (49, 53, 54). We believe that ours is the first recorded bloom of the genus Cyanothece. Other members of the Chroococcales, such as Synechococcus and Prochlorococcus, have been documented to form blooms in Florida Bay (6, 25, 38), Australian coastal lagoons (47), and also recorded in Brazilian coastal lagoons (12), but these are extremely small, picoplanktonic cells. Synechococcus, for example, seldom exceeds 2 μm in diameter, and even at its largest has a surface area/volume (SA/V) ratio of approximately 1.5, whereas the cells found in Lake St. Lucia had a biovolume about 25 times that of Synechococcus and an SA/V ratio of only 0.89. We suggest that the nutrient uptake dynamics of nanoplanktonic cells must be very different and that the high uptake efficiencies of the picoplanktonic cells, which result from their high SA/V ratio (1) and which account for their tendency to bloom even at low nutrient levels, do not apply in this case. The nutrient dynamics of our isolate remain the subject of ongoing research, but it should be noted that this is a mesotrophic system (37) and that it is likely that nutrient transfer from sediments to the very shallow water is extremely efficient, especially during windy periods, when rapid water movement across the sediments is seen. It must be borne in mind, however, that halotolerant cells may become nutrient stressed even at relatively high nutrient levels as salinity rises (31, 33), and thus cellular metabolic demands at high salinities may be much greater.
The cause of the onset of the bloom thus remains unclear, but we believe that the loss of grazing organisms at high salinity was the most significant factor in establishment of the bloom. An analogous situation, albeit for a protistan population, has been described by Buskey et al. (7, 8) and Stockwell et al. (49) for the Laguna Madre, an estuarine system in Texas also characterized by periodic development of hypersaline conditions. There, the onset of an 8-year bloom of Aureoumbra lagunensis was ascribed to the collapse of grazer populations due to the onset of hypersaline conditions as well as a fish kill due to cold weather, and its persistence was due to elimination of competitors. After 8 years, the bloom collapsed when salinities fell below 20 after a period of heavy rain. At this time, a complex planktonic community of ciliates, dinoflagellates, and zooplankters was reestablished, only to be lost again as hypersalinity reestablished and the Aureoumbra bloom returned to prominence (7, 8). We suggest that a similar dynamic operates in the northern areas of Lake St. Lucia. A further, telling similarity between the two systems is that Buskey et al. (7, 8) suggested that slow water turnover is a major contributor to maintenance of the bloom. In Lake St. Lucia, not only are the northern reaches currently receiving minimal freshwater input, but low water levels have resulted in their physical separation from the lower reaches, where salinity is far lower (>35) (29).
Long-term climate prediction models for the KwaZulu-Natal region are pertinent to this discussion. Overall, it has been suggested that the area will become somewhat wetter, but the models also predict that the frequency and duration of extreme climate events, such as floods and droughts, will also increase (18, 30). If the status quo in St. Lucia is maintained and the main freshwater input, the Mfolozi River, is kept separate, as it is at present, it is likely that the frequency of lengthy hypersaline periods in the lake will increase, interrupted by periods of flood. These conditions would strongly favor the development of halotolerant blooms in the lake: if not of Cyanothece, then of other cyanobacteria or even of halotolerant protists, such as Dunaliella.
The Cyanothece bloom described here persisted throughout the northern reaches of Lake St. Lucia for 18 months as the dominant phytoplankter, in a system of markedly reduced trophic complexity. It appears, from our examination of the literature, that this was one of the most persistent cyanobacterial blooms in a natural system on record, serving as a marker of the biological crisis of hypersalinity in which this system is currently placed. The current very low salinities throughout the system are, unfortunately, a temporary respite for Lake St. Lucia. Without substantial and repeated opening of the estuary mouth, the onset of the lengthy winter dry seasons will again see the rapid reduction of water volumes, with concomitant rises in salinities. We wait, with interest, to see if these will be accompanied by the reappearance of the identified Cyanothece sp. cells.
ACKNOWLEDGMENTS
Funding for this project was provided by the National Research Foundation (NRF Pretoria grant number 71051).
We thank the iSimangaliso Wetland Park Authority and the staff and management of Ezemvelo KwaZulu-Natal Wildlife (EKZN Wildlife). We are particularly grateful to Ricky Taylor and Caroline Fox for their help with logistics and field collections. Guy Bate and Pat Smailes kindly provided samples from early collections undertaken in 2007 to 2009. Ursula Scharler, David Dyer, Nelson Miranda, Nicola Carrasco, Sarah Bownes, Katrin Tirok, Holly Nel, and Nasreen Peer are gratefully acknowledged for their help in the field and Jennifer Godlonton for lab work. Angus MacDonald provided invaluable assistance with analysis of molecular data. We also thank James Wesley-Smith and Priscilla Maartens of the UKZN electron microscopy unit for their help with TEM and light microscopy work.
Footnotes
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Agawin N. S. R., Duarte C. M., Agusti S. 2000. Response of Mediterranean Synechococcus growth and loss rates to experimental nutrient inputs. Mar. Ecol. Prog. Ser. 206:97–106 [Google Scholar]
- 2. Andersen R. A., Berges J. A., Harrison P. J., Watanabe M. M. 2005. Recipes for freshwater and seawater media, p. 429–538 In Andersen R. A. (ed.), Algal culturing techniques. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 3. Anthony E. E. 1931. A note on capsule staining. Science 73:319–320 [DOI] [PubMed] [Google Scholar]
- 4. Bate G. C., Smailes P. A. 2008. The response of the diatom flora of St. Lucia Lake and estuary, South Africa, to a severe drought. Afr. J. Aquat. Sci. 33:1–15 [Google Scholar]
- 5. Bianchi T. S., et al. 2000. Cyanobacterial blooms in the Baltic Sea: natural or human-induced? Limnol. Oceanogr. 45:716–726 [Google Scholar]
- 6. Boyer J. N., Kelble C. R., Ortner P. B., Rudnick D. T. 2009. Phytoplankton bloom status: chlorophyll a biomass as an indicator of water quality condition in the southern estuaries of Florida, USA. Ecol. Indic. 9S:S56–S67 [Google Scholar]
- 7. Buskey E. J., Liu H., Collumb C., Bersano J. G. F. 2001. The decline and recovery of a persistent Texas brown tide algal bloom in the Laguna Madre (Texas USA). Estuaries 24:337–346 [Google Scholar]
- 8. Buskey E. J., Wysor B., Hyatt C. 1998. The role of hypersalinity in the persistance of the Texas ‘brown tide' in the Laguna Madre. J. Plankton Res. 20:1553–1565 [Google Scholar]
- 9. Carrasco N. K., Perissinotto R., Pillay D. 2010. Zooplankton of the St. Lucia Estuary during the current drought cycle: a comparison between open- and closed-mouth conditions. Mar. Ecol. Prog. Ser. 399:157–171 [Google Scholar]
- 10. Cepák V. 1996. Nucleoid morphology in the coccal cyanophyte Cyanothece halobia (Chroococcales, Cyanophyta): a DAPI fluorescence study. Phycologia 35:523–527 [Google Scholar]
- 11. Chi Z., Su C. D., Lu W. D. 2007. A new exopolysaccharide produced by marine Cyanothece sp. 113. Bioresour. Technol. 98:1329–1332 [DOI] [PubMed] [Google Scholar]
- 12. Clementino M. M., et al. 2008. Prokaryotic diversity in one of the largest hypersaline coastal lagoons in the world. Extremophiles 12:595–604 [DOI] [PubMed] [Google Scholar]
- 13. da Rosa C. E., et al. 2005. Cyanobacterial blooms in estuarine ecosystems: characteristics and effects on Laonereis acuta (Polychaeta, Nereididae). Mar. Pollut. Bull. 50:956–964 [DOI] [PubMed] [Google Scholar]
- 14. Decho A. W., Lopez G. R. 1993. Exopolymer microenvironments of microbial flora: multiple and interactive effects on trophic relationships. Limnol. Oceanogr. 38:1633–1645 [Google Scholar]
- 15. De Philippis R. D., Margheri M. C., Materassi R., Vincenzini M. 1998. Potential of unicellular cyanobacteria from saline environments as exopolysaccharide producers. Appl. Environ. Microbiol. 64:1130–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Philippis R., Margheri M. C., Pelosi E., Ventura S. 1993. Exopolysaccharide production by a unicellular cyanobacterium isolated from a hypersaline habitat. J. Appl. Phycol. 5:387–394 [Google Scholar]
- 17. Duguid J. P. 1951. The demonstration of bacterial capsules and slime. J. Pathol. Bacteriol. 63:673. [DOI] [PubMed] [Google Scholar]
- 18. Fauchereau N., Trzaska S., Roault M., Richard Y. 2003. Rainfall variability and changes in Southern Africa during the 20th century in the global warming context. Nat. Hazards 29:139–154 [Google Scholar]
- 19. Fielding A. T., Forbes A. T., Demetriades N. T. 1991. Chlorophyll concentrations and suspended particulate loads in St. Lucia, a turbid estuary on the east coast of South Africa. Afr. J. Mar. Sci. 11:491–498 [Google Scholar]
- 20. Forbes A. T., Cyrus D. P. 1993. Biological effects of salinity gradient reversals in a southeast African estuarine lake. Neth. J. Aquat. Ecol. 27:483–488 [Google Scholar]
- 21. Galat D. L., Verdin J. P. 1989. Patchiness, collapse and succession of a cyanobacterial bloom evaluated by synoptic sampling and remote sensing. J. Plankton Res. 11:925–948 [Google Scholar]
- 22. Garcia-Pichel F., Nübel U., Muyzer G. 1998. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch. Microbiol. 169:469–482 [DOI] [PubMed] [Google Scholar]
- 23. Grindley J. R., Heydorn A. E. F. 1970. Red water and associated phenomena in St. Lucia. S. Afr. J. Sci. 66:210–213 [Google Scholar]
- 24. Guillard R. R. L., Sieracki M. S. 2005. Counting cells in cultures with the light microscope, p. 239–252 In Andersen R. A. (ed.), Algal culture techniques. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 25. Hitchcock G. E., Brand L., Morrison D. 2007. Plankton blooms, p. 77–91 In Hunt J. H., Nuttle W. (ed.), Florida Bay science program: a synthesis of research on Florida Bay. Fish and Wildlife Research Institute Technical Report TR-11 Fish and Wildlife Research Institute, St. Petersburg, FL [Google Scholar]
- 26. Komárek J. 1976. Taxonomic review of the genera Synechocystis SAUV.1892, Synechococcus NAG.1849, and Cyanothece gen. nov. (Cyanophyceae). Arch. Protist. 118:119–179 [Google Scholar]
- 27. Komárek J., Anagnostidis K. 1986. Modern approach to the classification system of cyanophytes. 2. Chroococcales. Arch. Hydrobiol. Algolog. Studies 43(Suppl. 73):157–226 [Google Scholar]
- 28. Komárek J., Cepák V. 1998. Cytomorphological characters supporting the taxonomic validity of Cyanothece (Cyanoprokaryota). Plant Syst. Evol. 210:25–39 [Google Scholar]
- 29. Lawrie R. A., Stretch D. D. 2011. Anthropogenic impacts on the water and salt budgets of St. Lucia estuarine lake in South Africa. Estuar. Coast. Shelf Sci. 93:58–67 [Google Scholar]
- 30. Lumsden T. G., Schulze R. E., Hewitson B. C. 2008. Evaluation of potential changes in hydrologically relevant statistics of rainfall in Southern Africa under conditions of climate change. Water SA 35:649–656 [Google Scholar]
- 31. Marcarelli A. M., Wurtsbaugh W. A., Griset O. 2006. Salinity controls phytoplankton response to nutrient enrichment in the Great Salt Lake, Utah, USA. Can. J. Fish. Aquat. Sci. 63:2236–2248 [Google Scholar]
- 32. Margheri M. C., Bosco M., Giovannetti L., Ventura S. 1999. Assessment of the genetic diversity of halotolerant coccoid cyanobacteria using amplified 16S rDNA restriction analysis. FEMS Microbiol. Lett. 173:9–16 [Google Scholar]
- 33. Moisander P. H., McClinton E., III, Paerl H. W. 2002. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb. Ecol. 43:432–442 [DOI] [PubMed] [Google Scholar]
- 34. Paerl H. W. 1996. A comparison of cyanobacterial bloom dynamics in freshwater, estuarine and marine environments. Phycologia 35:25–35 [Google Scholar]
- 35. Paerl H. W. 1988. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol. Oceanogr. 33:823–847 [Google Scholar]
- 36. Paerl H. W., Huisman J. 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 1:27–37 [DOI] [PubMed] [Google Scholar]
- 37. Perissinotto R., Pillay D., Bate G. 2010. Microalgal biomass in the St. Lucia Estuary during the 2004 to 2007 drought period. Mar. Ecol. Prog. Ser. 405:147–161 [Google Scholar]
- 38. Philps E. T., Badylak S., Lynch T. C. 1999. Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a sub-tropical inner-shelf lagoon. Limnol. Oceanogr. 44:1166–1175 [Google Scholar]
- 39. Pillay D., Perissinotto R. 2008. The benthic macrofauna of the St. Lucia estuary during the 2005 drought year. Estuar. Coast. Shelf Sci. 77:35–46 [Google Scholar]
- 40. Pillay D., Perissinotto R. 2009. Community structure of epibenthic meiofauna in the St. Lucia estuarine lake during a drought cycle. Estuar. Coast Shelf Sci. 81:94–104 [Google Scholar]
- 41. Pliński M., Mazur-Marzec H., Józwiak T., Kobos J. 2007. The potential causes of cyanobacterial blooms in Baltic Sea estuaries. Int. J. Oceanogr. Hydrobiol. 36:125–137 [Google Scholar]
- 42. Porta D., Rippka R., Hernández-Mariné M. 2000. Unusual ultrastructural features in three strains of Cyanothece (cyanobacteria). Arch. Microbiol. 173:154–163 [DOI] [PubMed] [Google Scholar]
- 43. Reddy K. J., Haskell J. B., Sherman D. M., Sherman L. A. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 175:1284–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reynolds E. S. 1963. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 17:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roussomoustakaki M., Anagnostidis K. 1991. Cyanothece halobia, a new planktic chroococcalean cyanophyte from Hellenic heliothermal saltworks. Arch. Hydrobiol. Suppl. Algolog. Stud. 64:71–95 [Google Scholar]
- 46. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 47. Schapira M., Buscot M.-J., Pollet T., Leterme S. C., Seuront L. 2010. Distribution of picophytoplankton communities from brackish to hypersaline communities in a South Australian lagoon. Saline Systems 6:2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spurr A. R. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31–43 [DOI] [PubMed] [Google Scholar]
- 49. Stockwell D. A., Buskey E. J., Whitledge T. E. 1993. Studies on conditions conducive to the development and maintenance of a persistent “brown tide” in Laguna Mardre, Texas, p. 693–698.In Smayda T. J., Shimizu Y. (ed.), Toxic phytoplankton blooms in the sea. 5th International Conference on Toxic Marine Phytoplankton Elsevier, Amsterdam, Netherlands [Google Scholar]
- 50. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4. Mol. Biol. Evol. 24:1569–1599 [DOI] [PubMed] [Google Scholar]
- 51. Tamura K., Nei M., Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U. S. A. 101:11030–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor R. H., Adams J. B., Haldorsen S. 2006. Primary habitats of the St. Lucia estuarine system, South Africa, and their responses to mouth management. Afr. J. Aquat. Sci. 31:31–41 [Google Scholar]
- 53. Van Rijn J., Cohen Y. 1983. Ecophysiology of the cyanobacterium Dactylococcopsis salina: effect of light intensity, sulphide and temperature. J. Gen. Microbiol. 129:1849–1856 [Google Scholar]
- 54. Walsby A. E., Van Rijn J., Cohen Y. 1983. The biology of a new gas-vacuolate cyanobacterium, Dactylococcopsis salina sp. nov., in Solar Lake. Proc. Roy. Soc. Lond. B Biol. Sci. 217:417–447 [Google Scholar]
- 55. Waterbury J. B. 2006. The cyanobacteria: isolation, purification, and identification, p. 1053–1073 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes: a handbook on the biology of bacteria, vol. 4 Springer, New York, NY. [Google Scholar]
- 56. Waterbury J. B., Rippka R. 1989. The order Chroococcales Wettstein 1924, emend. Rippka et al., 1979, p. 1728–1746 In Staley J. T., Bryant M. P., Pfennig N., Holts J. G. (ed.), Bergey's manual of systematic bacteriology, vol. 3 Williams and Wilkins, Baltimore, MD [Google Scholar]
- 57. Welschmeyer N. A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and phaeopigments. Limnol. Oceanogr. 39:1985–1992 [Google Scholar]
- 58. Whitfield A., Taylor R. H. 2009. A review of the importance of freshwater inflow to the future conservation of Lake St. Lucia. Aquat. Conserv. Mar. Freshw. Ecoyst. 19:838–848 [Google Scholar]