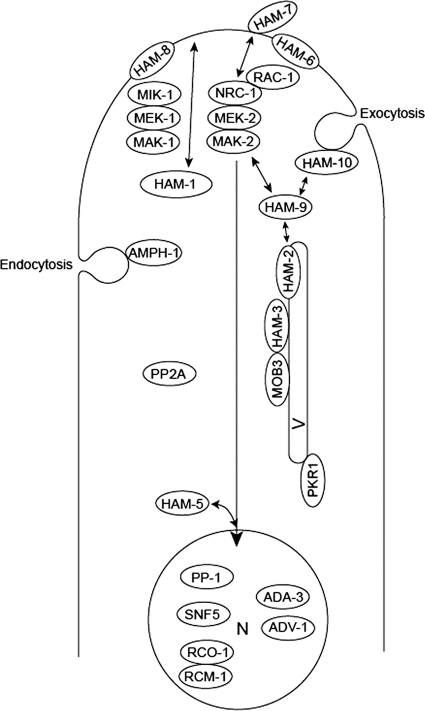
Fig. 6.
Schematic representation of the functions proposed for cell fusion proteins. The transcription factor PP-1 is a target for phosphorylation by MAK-2. PP-1, along with the other transcription factors identified (ADV-1, ADA-3, SNF5, and the dimeric RCO-1/RCM-1), is proposed to reside in the nucleus (N) and to direct the transcription of the cell fusion genes. HAM-5 is proposed to function in directing MAK-2 into the nucleus, where it phosphorylates PP-1 and perhaps some of the other transcription factors. In addition to regulating transcriptional activity, the two MAP kinase signaling pathways (NRC-1/MEK-2/MAK-2 and MIK-1/MEK-1/MAK-1) also regulate CAT formation and growth by controlling vesicular trafficking to and from the hyphal tip. The two MAP kinase pathways are activated by cell-to-cell signaling, and the plasma membrane-associated HAM-6, HAM-7, and HAM-8 proteins might function as receptors for the signaling factor(s) or might be directly involved in fusion between the plasma membranes. As part of the cell-to-cell signaling process, the HAM-1/SO protein and the RAC-1/NRC-1/MEK-2/MAK-2 complex transiently associate with the plasma membrane in an oscillatory “ping pong” manner. The PP2A phosphatase might function in dephosphorylating the MAP kinase pathway proteins. The HAM-2/HAM-3/MOB3 complex is located on an intracellular vesicle (V) and is proposed to regulate vesicular trafficking to the hyphal tip. We propose that the HAM-2/HAM-3/MOB3 complex could receive input from the MAP kinase pathways and that the HAM-9 protein could function to connect the HAM-2/HAM-3/MOB3 complex to the MAP kinase pathways. AMPH-1 is proposed to play roles in mediating the formation of endosomes and to be necessary to maintain a balance between exocytosis and endocytosis of vesicles during CAT growth. The HAM-10 protein may function in regulating the movement of vesicles at the hyphal tip. The PKR1 protein is found in the ER and is necessary for efficient vesicular trafficking in the secretory pathway.