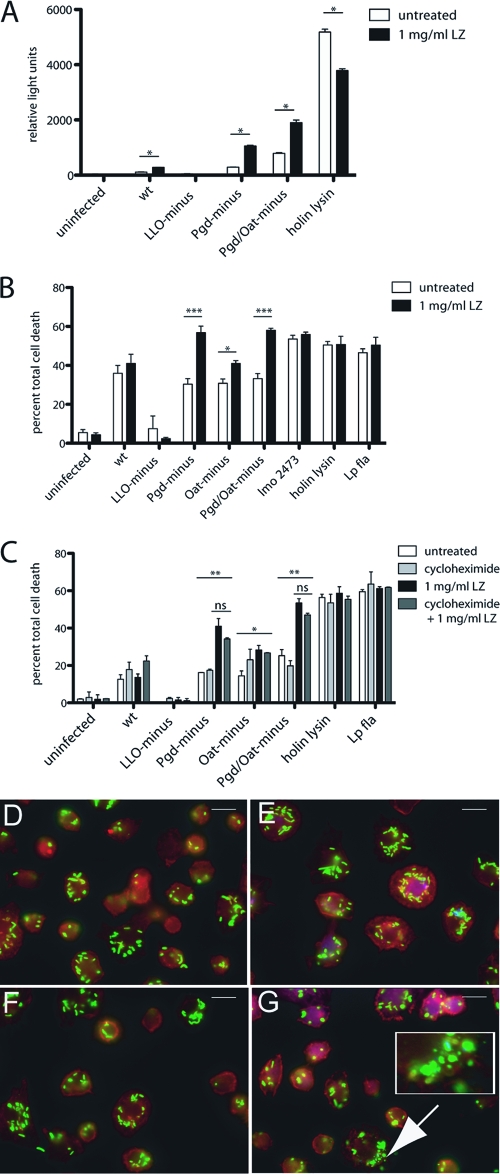
Fig. 8.
Extracellular lysozyme accesses the BMM cytosol and results in increased degradation of lysozyme-sensitive L. monocytogenes strains and subsequent host cell death. (A) IFN-α/βR− BMM were infected with L. monocytogenes bearing a plasmid-containing luciferase under a CMV promoter, and 1 mg/ml extracellular lysozyme was added at 1 h postinfection. Luciferase expression was detected at 6 h postinfection. (B) Pam3CSK4-stimulated LysM− BMM were infected at an MOI of 5, and 1 mg/ml extracellular lysozyme was added at 1 h postinfection. LDH release was measured at 6 h. (C) Pam3CSK4-stimulated LysM− BMM were treated with cycloheximide and infected at an MOI of 5, and LDH release was measured at 6 h. Error bars represent standard deviations of the means. (A and B) Statistical significance was evaluated using Student's t test. *, P < 0.05; ***, P < 0.0001. (C) One-way ANOVA was performed to analyze the statistical significance of LDH release following various treatments of BMM infected with individual L. monocytogenes strains. *, P < 0.05; **, P < 0.001. The means of LDH values from infections treated with lysozyme compared to those treated with lysozyme plus cycloheximide were determined to be not significant (ns) using Student's t test. The means of LDH values from infections with Pgd−, Oat−, or Pgd− Oat− L. monocytogenes were significant by one-way ANOVA. Data are representative of at least 3 independent experiments with similar results. LysM− BMM were infected with either wt (D, E) or Pgd− Oat− (F, G) L. monocytogenes for 2 h. A total of 1 mg/ml extracellular lysozyme was added at 1 h postinfection (E, G), and cells were scored for evidence of bacterial degradation (depicted in inset). Size bars represent 10 μm. Data are representative of more than 3 independent experiments with similar results.