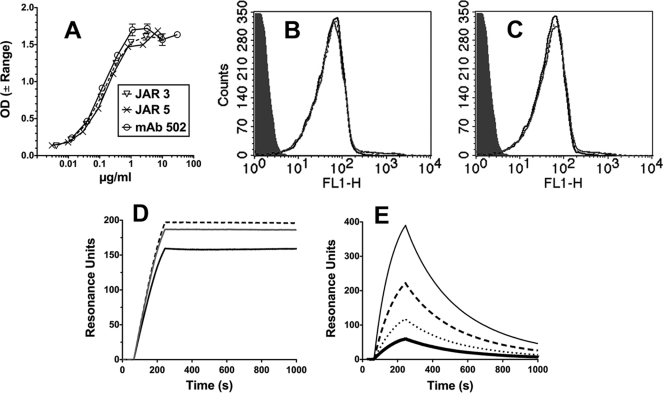
Fig. 1.
Binding of anti-fHbp MAbs. (A) ELISA. IgG bound to immobilized fHbp was detected with an anti-human kappa light-chain-specific alkaline phosphatase-conjugated antibody. Error bars represent the range in OD values observed in two independent experiments. (B and C) Flow cytometry. (B) Binding of anti-fHbp MAbs (4 μg/ml) with live bacterial cells of N. meningitidis group B strain H44/76. JAR 3, black dashed line; JAR 5, gray line; MAb502, black line. An irrelevant human MAb (100 μg/ml) served as a negative control (gray filled histogram). The binding curves of the three anti-fHbp MAbs are superimposed. (C) Same MAb concentrations as in panel B in the presence of heat-inactivated 20% IgG-depleted human serum as a source of human fH (∼90 μg/ml). (D and E) Surface plasmon resonance. (D) Representative data for binding of 0.25 μg/ml anti-fHbp MAbs (1.7 nM) to immobilized recombinant fHbp (1,000 RU). Lines are as in panel B. (E) Binding of purified human fH to immobilized recombinant fHbp (1,000 RU). fH concentrations of 12 to 90 μg/ml (71 to 580 nM) are shown: 12 μg/ml, thick black line; 23 μg/ml, dotted black line; 45 μg/ml, dashed black line; and 90 μg/ml, gray solid line. The flow cytometric data were replicated in two or three independent experiments.