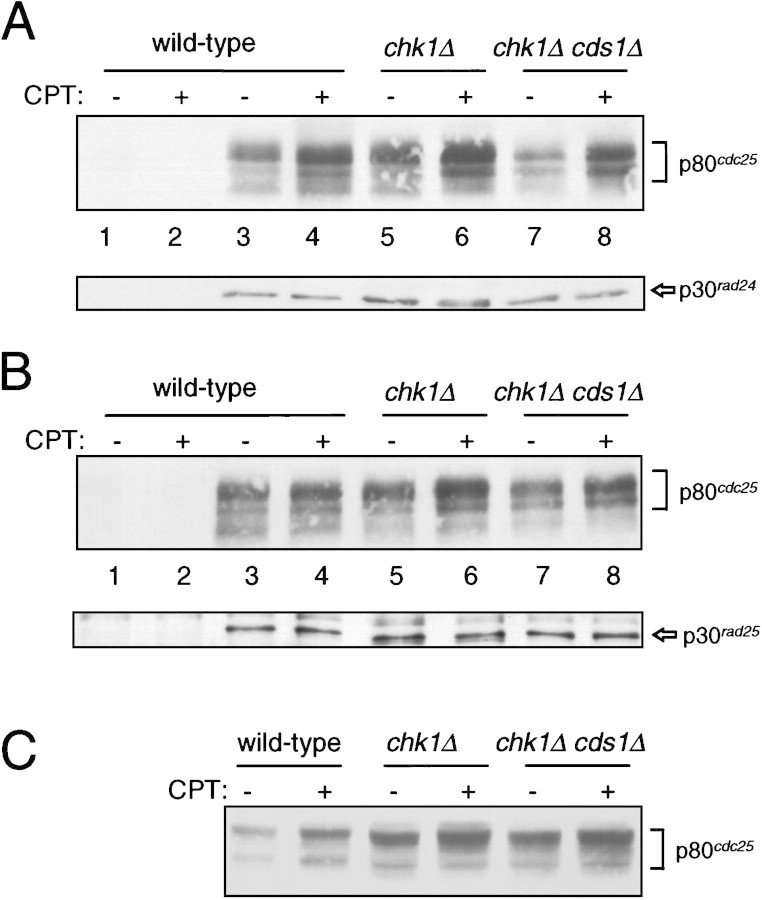
Figure 7.
Association of Cdc25 with 14-3-3 proteins before and after DNA damage is not dependent on Chk1 or Cds1. Strains with integrated cdc25:3HA were exposed to 40 μm CPT for 2 hr. Lysates were prepared and subjected to immunoprecipitation with antibody to Rad24 (UMDNJ-55) and Rad25 (UMDNJ-56). (A) Rad24 immunoprecipitates were separated by SDS-PAGE and immunoblotted with antibody to detect Cdc25 (12CA5, top) and Rad24 (UMDNJ-55, bottom). The samples in lanes 1 and 2 are precipitates of wild-type cells with preimmune sera. The samples in lanes 3–8 are precipitates with Rad24 antisera (UMDNJ-55). (Lanes 3,4 Wild-type cells (BN71); (lanes 5,6) chk1Δ cells (NW291); (lanes 7,8) chk1Δ cds1Δ cells (NW373). (B) Rad25 immunoprecipitates were separated by SDS-PAGE and immunoblotted with antibody to detect Cdc25 (12CA5, top) and Rad25 (UMDNJ-55, bottom). The samples in lanes 1 and 2 are precipitates of wild-type cells with preimmune sera. The samples in lanes 3–8 are precipitates with Rad25 antisera (UMDNJ-56). (Lanes 3,4) Wild-type cells (BN71); (lanes 5,6) chk1Δ cells (NW291); (lanes 7,8) chk1Δ cds1Δ cells (NW373). (C) Samples of the lysates used for immunoprecipitation in A and B were immunoblotted for Cdc25 protein with mAb12CA5. The amount of Cdc25 in the lysates is increased no more than twofold by DNA damage (estimated by Western blot analysis of serial dilutions of the samples from wild-type cells).