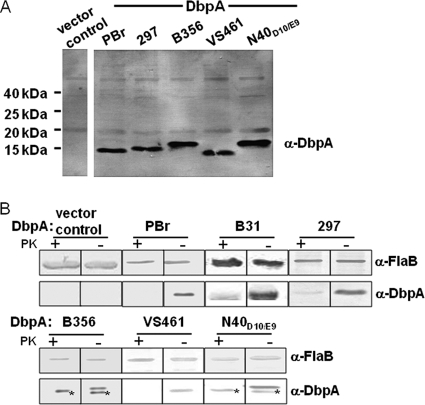
Fig. 2.
DbpA alleles from diverse Lyme disease spirochetes are expressed on the surface of a nonadherent B. burgdorferi strain. (A) Triton X-114 extractions of B. burgdorferi strain B314 expressing the indicated DbpA allele were subjected to SDS-15% PAGE and immunoblotted with a mixture of antisera raised against DbpAB31, DbpAN40-D10/E9, DbpAPBr, and DbpAVS461. (B) Spirochetes expressing DbpA protein were digested with proteinase K (PK+) or incubated in PBS (PK−). Lysates from 5 × 107 treated bacteria were separated by SDS–15% PAGE, and DbpA and FlaB proteins were identified by Western blotting with an individual antiserum against DbpAN40-D10/E9, DbpAB31, DbpAPBr, or DbpAVS461. The proteinase K sensitivity experiments were performed several times and Fig. 2 is representative of such studies. Proteinase K-resistant species in lysates of B314/pDbpAB356 and B314/pDbpAN40-D10/E9, indicated by asterisks, were consistently observed and may represent intracellular nonlipidated DbpA protein. Flagellin, a periplasmic protein, was immunoblotted as a control for the retention of outer membrane integrity. B. burgdorferi strain B314 expressing DbpAB31 was analyzed in a separate experiment.