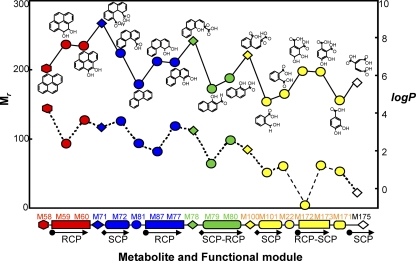
Fig. 4.
Relationships between nodes (chemical compounds) and their molecular weights (Mr) and water solubility (log P) on the network. Modularity does not always guarantee clear-cut subnetworks linked in well-defined ways, but there is a high degree of overlap and cross-talk between modules (8). As degradation proceeds, metabolites repeatedly move up and down in molecular weight and hydrophobicity, which is in accordance with the functional modules in the degradation process. This repeating pattern of the chemical properties over the degradation process strongly supports the concept of functional modules in the PAH-MN of M. vanbaaleniiPYR-1. Hexagons, circles, and rhombuses indicate starting PAH, intermediates, and ring cleavage metabolites, respectively. The color denotes the number of aromatic rings: red, 4; blue, 3; green, 2; yellow, 1; white, 0. Numbers with M represent PAHs and their metabolites, which can be found in Fig. 2and Table S4 in the supplemental material. The log Pvalues for the water solubility of the compounds were calculated by MarvinSketch version 4.1.8.