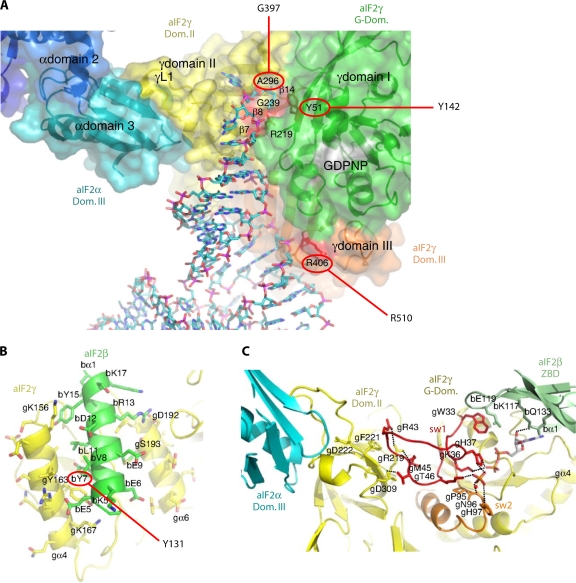
Fig. 11.
Interfaces between subunits of archaeal aIF2 and docking tRNA on aIF2γ. (A) Model of Phe-tRNAPhe (in a stick figure) docked onto the S. solfataricus aIF2αγ heterodimer. aIF2γ residues corresponding to those in yeast eIF2γ implicated in tRNA binding by the Sui− substitutions Y142H and G397A are circled. See the text for more details. (Reproduced from reference 223 with slight modifications, with permission from Elsevier.) (B and C) Segments of aIF2α, aIF2γ, and aIF2β at the subunit interfaces of the S. solfataricus heterotrimer of full-length β and γ subunits and domain III of aIF2α. Residues are labeled with a prefix (a, b, or g) indicating their subunit origin (α, β, or γ). (B) Helix α1 in aIF2β is wedged between two helices in the G domain of aIF2γ. The aIF2β residue corresponding to the yeast Sui− substitution Y131 is circled. (C) sw1 in the G domain interacts directly with residues in the ZBD of aIF2β and with domain II of aIF2γ. The ZBD also contacts the ribose ring of the bound GDP (shown in a stick figure). Several ZBD residues near the interface with sw1 are immediately adjacent to residues corresponding to yeast eIF2β residues altered by Sui− substitutions that confer increased GTP hydrolysis by the TC. (Panels B and C are reproduced from reference 224 with slight modifications, with permission of the publisher [copyright 2007 National Academy of Sciences, U.S.A.].)