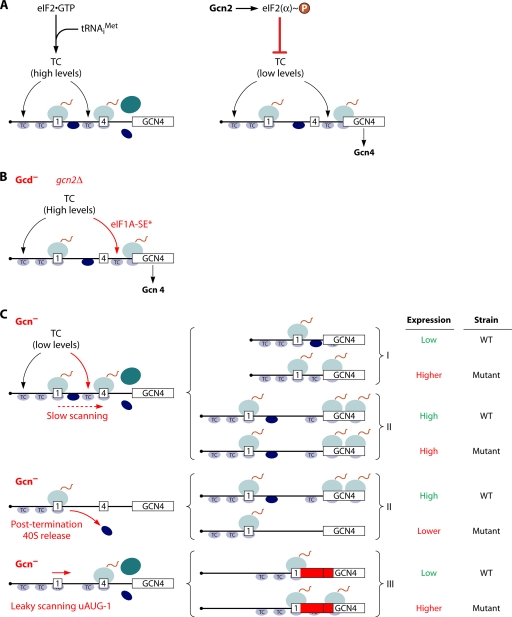
Fig. 3.
Schematic model of GCN4 translational control and mechanisms of Gcd− and Gcn− mutations. (A) Following the translation of uORF1 (boxed 1), posttermination 40S subunits remain attached to the GCN4 mRNA and resume scanning. Under nonstarvation conditions (left), they quickly rebind the TC and reinitiate at uORF4 (boxed 4), and the 80S ribosome dissociates after terminating at uORF4. Under amino acid starvation conditions (right), the concentration of the TC is reduced by eIF2α phosphorylation, such that many 40S ribosomes fail to rebind the TC until scanning past uORF4 and can reinitiate at the GCN4 ORF instead. (B) GCN4 translation is normally constitutively repressed in gcn2Δ cells owing to the inability to phosphorylate eIF2α in response to starvation. However, mutations that reduce the rate of TC loading on 40S subunits, such as substitutions in the eIF1A SE elements (eIF1A-SE*) (see text for details), constitutively derepress GCN4 translation, producing the Gcd− phenotype. (C, left) Defects in different steps of reinitiation pictured here all prevent the induction of GCN4 translation in starved cells despite the reduction in TC levels, conferring the Gcn− phenotype. Mechanisms of Gcn− phenotypes can be discerned by analyzing the expression of the solitary uORF1 constructs depicted on the right. Gcn− mutations that confer slow scanning should increase reinitiation at GCN4 for construct I, in which uORF1 is very close to the GCN4 uORF, by increasing the scanning time available for the reassembly of the PIC, but have little effect on construct II, in which uORF1 is far upstream of GCN4, and the scanning time available for reinitiation is long. Gcn− mutations that confer the release of posttermination 40S subunits or reduce their ability to resume scanning will reduce the expression of constructs I and II. Gcn− mutations that confer a leaky scanning of uORF1 will increase expression from construct III, in which uORF1 is elongated and extensively overlaps the GCN4 ORF, abolishing reinitiation.