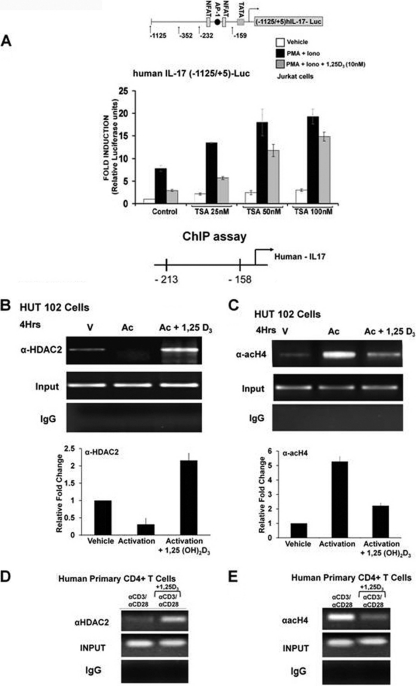
Fig. 5.
1,25(OH)2D3 recruits histone deacetylase to the hIL-17A promoter. (A) Jurkat cells were transfected with the −1125/+5 hIL-17A promoter. Posttransfection cells were rested overnight and then stimulated with PMA and ionomycin in the presence or absence of 1,25(OH)2D3 (10 nM) for 9 h with the indicated concentrations of HDAC inhibitor, trichostatin A (TSA). P < 0.01 [activation plus 1,25(OH)2D3, control versus activation plus 1,25(OH)2D3, TSA (50 and 100 nM)]. Data represent the means ± SE from 3 separate experiments. (B to E) 1,25(OH)2D3 recruits histone deacetylase to the hIL-17A. (B and C) ChIP assays were performed using HUT102 cells. HUT102 cells were stimulated with PMA and ionomycin in the presence or absence of 1,25(OH)2D3 (10 nM) for 4 h and cross-linked with 1% formaldehyde for 15 min. Cross-linked lysates were prepared and subjected to immunoprecipitation with HDAC-2 antibody (B), AcH4 antibody (C), or control rabbit IgG (B and C). DNA precipitates were isolated and then subjected to PCR using specific primers designed to amplify the −213/−158 region of the hIL-17A promoter, which contains NFAT sites. Analysis of input DNA (0.2%) was taken prior to precipitation. The bar graph represents quantification for HUT102 cells of three independent ChIP analyses (±SE). (D and E) ChIP assays were performed using CD4+ T cells polarized under Th17 conditions. Human CD4+ T cells were activated in the presence or absence of 10 nM 1,25(OH)2D3 for 4 days. Formaldehyde cross-linked lysates were subjected to immunoprecipitation with HDAC2 antibody (D), AcH4 antibody (E), or control rabbit IgG (D and E). DNA precipitates were isolated and subjected to PCR as described above. Similar results were observed in two additional experiments.