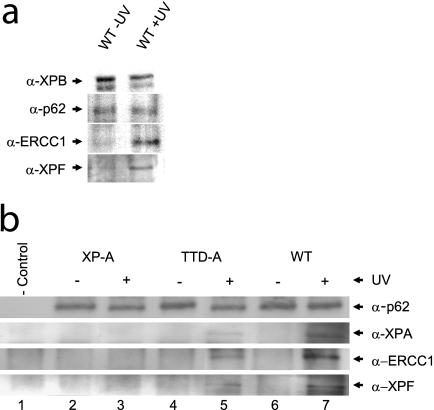
Fig. 4.
Recruitment of NER proteins to chromatin after UV irradiation. (a) Western on ChIP was performed on extracts isolated after formaldehyde cross-linking of wild-type (MRC5-SV) cells (with and without 25 J/m2 UV irradiation; 1 h after irradiation) using an XPB-specific rabbit polyclonal antibody. Equal amounts of precipitated TFIIH were loaded (analyzed by anti-XPB and anti-p62 immunostaining [top two rows]). Under these conditions, a strong increase in the NER endonuclease complex ERCC1-XPF (bottom two rows) was observed to coprecipitate with chromatin-bound TFIIH after UV irradiation. (b) Western on ChIP analysis of XP-A (XP2OS-SV, lanes 2 and 3), TTD-A (TTD1BR-SV, lanes 4 and 5), and wild-type (MRC5-SV, lanes 6 and 7) cells from UV-treated (+) or mock-treated (−) cells, as in panel a. Equal amounts of precipitated TFIIH (anti-p62 signal) were loaded. The immunoprecipitated material was probed for XPA, ERCC1, and XPF and compared to nonspecific precipitated material (lane 1) from preimmune rabbit IgG. In XP-A cells, XPA, ERCC1, and XPF do not coprecipitate after UV irradiation, whereas in TTD-A cells, strongly reduced amounts of these proteins were coprecipitated with TFIIH after UV irradiation.