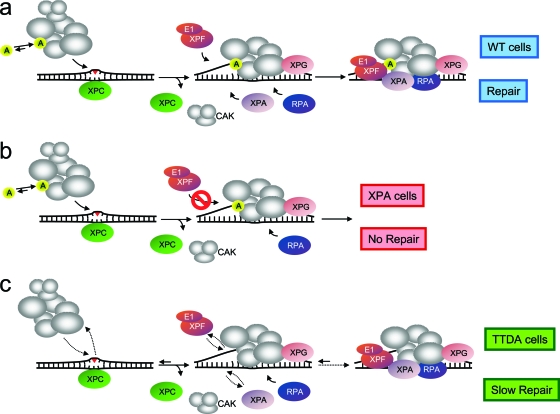
Fig. 5.
Model for TTDA function in NER and proposed model for lesion-bound NER complex formation in wild-type, XP-A, and TTD-A cells. (a) In wild-type cells, XPC binds to UV lesions and recruits TFIIH and XPG, followed by XPA binding via its interaction with TFIIH. Under these conditions, TTDA (p8) is more stably bound to TFIIH and promotes the subsequent steps in NER complex formation and possibly stimulates the helicase activity of TFIIH. The unwound DNA structure is stabilized by the presence of TFIIH, XPA, and RPA; together, these proteins are required for recruiting the ERCC1-XPF complex via XPA interaction. After assembling the complete NER machinery, dual incision can occur, and these intermediates are further processed by gap-filling synthesis and ligation. (b) In XP-A cells, recruitment of XPC, TFIIH, and XPG occurs normally, including stable association of TTD-A. Nevertheless, due to the absence of XPA, downstream NER factors were not assembled, and thus NER activity is completely abolished. (c) In TTD-A cells, TFIIH stability and probably protein conformation have been altered because of the complete absence or the presence of a truncated TTDA protein. Efficient binding of mutated TFIIH is affected and reduces its affinity for the NER complex. Retarded binding of mutated TFIIH attracts XPA to the site of damage to stabilize the NER complex. Furthermore, incorporation of ERCC1-XPF into the NER complex is also retarded similarly to XPA. Nevertheless, although retarded, the assembly of the different NER factors takes place, and finally, DNA lesions are repaired.