Abstract
The storage of biological samples may affect detection of viral nucleic acid, yet the stability of viral nucleic acid at standard laboratory storage temperatures (−70°C and −20°C) has not been comprehensively assessed. Deterioration of viral RNA and DNA during storage may affect the detection of viruses, thus leading to an increased likelihood of false-negative results on diagnostic testing. The viral loads of 99 hepatitis C virus (HCV), 41 HIV, and 101 hepatitis B virus (HBV) patient samples were measured before and after storage at −20°C and −70°C for up to 9.1 years using Versant branched DNA assays, Cobas Monitor assays, and/or AmpliPrep/AmpliScreen assays. Clinical samples stored at −20°C for up to 1.2 years and at −70°C for up to 9 years showed a statistically significant difference from baseline with respect to HCV RNA titer, although this difference was not greater than 0.5 log10 unit. The concentration of HIV RNA in clinical samples stored at −20°C for 2.3 years and at −70°C for up to 9.1 years did not differ significantly from the baseline viral load. HBV DNA-positive clinical samples stored at −20°C for up to 5 years and at −70°C for up to 4 years differed significantly in viral load. In all studies, however, the loss of viral load of HCV, HIV, or HBV in clinical samples tested after storage at −20°C and −70°C for up to 9 years ranged from 0.01 to 0.35 log10 IU/ml and did not exceed 0.5 log10, which is the estimated intra-assay variation for molecular tests. Hence, the loss was considered of minimal clinical impact and adequate for the detection of HCV, HIV-1, and HBV nucleic acids using nucleic acid assays for the assessment of the infectious risk of cell, blood, and tissue donors.
INTRODUCTION
Biological samples stored for long time periods are increasingly being utilized as therapeutic agents, for example, for cord blood allografts in children with hematological malignancy (3) and bone allografts during reconstructive surgery (12). The safety of these allografts with respect to transmission of the blood-borne viruses (BBVs), human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), needs to be accurately assessed, often years after the sample was collected. This requires confidence that the result of the nucleic acid test (NAT) used will be accurate and is dependent upon (i) the robustness of the assay (18), (ii) the sensitivity of the NAT, and (iii) the stability of the target nucleic acid, which must not have deteriorated significantly during handling and storage of the samples. Many authors report that storage conditions of samples may affect nucleic acid stability, and, hence, the ability to detect viral nucleic acid, especially detection of low viral concentrations, could be compromised (2, 4, 10, 11, 13, 14). The stability of viral nucleic acid (RNA and DNA) under standard storage conditions (−70°C and −20°C) becomes critical for assessing the infectious status of cells, tissues, or organs being used therapeutically, where the nucleic acid concentrations are low, where the samples are stored for many years, or as is the case for nearly all commercial assays, where the NATs are validated for specimens stored for up to 1 month only. Viral DNA is more resistant to degradation under storage conditions since DNA is, in principle, more stable than RNA (7). In order to ensure that deterioration of viral RNA and DNA during storage does not affect the detectability of viruses, we evaluated the stability of viral RNA and DNA of clinical samples known to be positive for HIV, HCV, or HBV stored at −20°C and −70°C for up to 9 years. The results support the adequacy of these previously nonconformant samples for detection of HIV, HCV, and HBV nucleic acid, using commercial assays in the area of regulated, licensed testing, in blood from donors of biological therapeutics such as cord blood (1) and bone allografts (21).
MATERIALS AND METHODS
Plasma samples were stored following initial baseline testing at −20°C and/or −70°C in routinely monitored freezers. Samples were tested either at the South Eastern Area Laboratory Service (SEALS), Department of Microbiology at the Prince of Wales Hospital (Sydney, Australia), or the Victorian Infectious Diseases Reference Laboratory (VIDRL; Melbourne, Australia) with assays performed by different operators due to the time span of the study.
Stability of HCV RNA. (i) Stability of HCV RNA in samples stored for 1.2 years at −20°C and −70°C.
Seven plasma samples with HCV RNA levels of between 1 × 106 and 1 × 107 IU/ml were collected and tested using the Roche Cobas AmpliPrep/Cobas TaqMan HCV (CAP-CTM) assay (Roche Diagnostics, GmbH, Mannheim) on different dates. The plasma samples were diluted with fresh frozen plasma (FFP) from the SEALS Blood Bank known to be negative for HIV, HCV, and HBV by both serology and NATs. Three serial dilutions were performed with FFP: 1/100 for a final concentration of 104 IU/ml, 1/1,000 for a final concentration of 103 IU/ml, and 1/10,000 for a final concentration of 102 IU/ml.
Samples were initially tested using the Roche Cobas AmpliPrep/Cobas TaqMan HCV assay and subsequently diluted and divided into two aliquots, placed in two different cryoboxes, and allocated to one of the two storage protocols (−20°C or −70°C) for 1.2 years. Following 1.2 years of storage, aliquots of each concentration were thawed at ambient temperature for 1 h and tested immediately on the Roche Cobas AmpliPrep/Cobas TaqMan HCV assay. The limit of detection of this assay was 15 IU/ml, and the linear range was 43 IU/ml to 6.90 × 107 IU/ml.
(ii) Stability of HCV RNA in samples stored for 1 year at −20°C.
Thirty-five HCV RNA-positive plasma samples were tested using the Siemens Versant HCV RNA, version 3.0 (Versant HCV RNA 3.0) branched DNA [bDNA]) assay (Siemens Healthcare Diagnostics, Tarrytown, NY) and stored at −20°C for 1 year. After a year, the samples were collected, thawed, and retested using the same assay. The limit of detection of this assay was 615 IU/ml, and the linear range was 615 IU/ml to 7.69 × 106 IU/ml.
(iii) Stability of HCV RNA in samples stored for up to 9 years at −70°C.
Twenty-two HCV RNA-positive plasma samples were tested using the Roche Cobas Amplicor HCV Monitor (version 2.0) assay and stored at −70°C for up to 9 years; samples were thawed, and the viral load was measured using the same assay. The limit of detection of this assay was 600 IU/ml, and the linear range was 600 IU/ml to 7 × 105 IU/ml.
Stability of HIV-1 RNA. (i) Stability of HIV-1 RNA in samples stored for 2.3 years at −20°C.
Twenty-two HIV RNA-positive plasma samples were tested using the Siemens Versant HIV RNA 3.0 (bDNA) assay. Following initial testing, three different HIV RNA concentration levels of 102, 103, and 104 IU/ml were selected, and samples were stored for 2.3 years at −20°C. Subsequent to storage, these samples were thawed and retested using the same assay. The limit of detection of this assay was 50 copies/ml, and the linear range was 50 copies/ml to 5 × 105 copies/ml.
(ii) Stability of HIV-1 RNA in samples stored for up to 9.1 years at −70°C analyzed with Cobas Amplicor HIV-1 Monitor (version 1.5) assay.
Nineteen plasma samples positive for HIV RNA using the Roche Cobas Amplicor (version 1.5) assay (standard specimen and control preparation) were stored for up to 9.1 years at −70°C. The samples were thawed and then tested using the Roche Cobas Amplicor (version 1.5) assay with ultrasensitive specimens and the control preparation, which increases the sensitivity of the Cobas Amplicor (version 1.5) assay. The limit of detection of the standard specimens and control preparation was 400 copies of HIV RNA/ml, and the linear range was 400 copies/ml to 7.5 × 105 copies/ml. The limit of detection of the ultrasensitive specimens and control preparation was 50 copies of HIV RNA/ml, and the limit of detection was 50 copies/ml to 105 copies/ml.
Stability of HBV DNA. (i) Stability of HBV DNA in samples stored for 1.8 to 5 years at −20°C.
Thirty HBV DNA-positive plasma samples were tested using the Roche Cobas AmpliPrep/Cobas TaqMan assay and stored at −20°C for up to 1.8 years. Following storage, these samples were thawed and reanalyzed using the same assay. Another 40 serum samples positive for HBV DNA were tested at the VIDRL using the Siemens Versant HBV DNA 3.0 (bDNA) assay and subsequently reanalyzed using the same assay after 5 years of storage. The limit of detection of the Cobas AmpliPrep/Cobas TaqMan HBV assay was 12 IU/ml, and the linear range was 20 IU/ml to 1.7 × 108 IU/ml, and the limit of detection of the Versant HBV DNA 3.0 (bDNA) assay was 351 IU/ml HBV DNA, and the linear range was 3.51 × 102 to 1.8 × 107 HBV DNA IU/ml.
(ii) Stability of HBV DNA in samples stored for up to 4 years at −70°C.
Thirty-one HBV DNA-positive serum samples previously tested at VIDRL using the HBV bDNA assay were stored at −70°C for up to 4 years and reanalyzed using the same assay.
Statistical analysis.
The differences of the log10-transformed HCV RNA, HIV RNA, and HBV DNA loads before and after the storage period were calculated. The 95% confidence intervals (CIs) were calculated with the mean and the standard deviation (SD) for the log difference. The mean ± SD was plotted into figures using GraphPad Prism software (version 5 for Windows; GraphPad Software, San Diego, CA). The Student's t test (2-tailed) was used to determine significance between the mean difference in viral load following storage at −20°C and −70°C for HCV, HIV, and HBV, where a P value lower than 0.05 was considered significant. However, if the two-sided 95% CI of the RNA/DNA difference before and after storage was within ±0.5 log10 unit, then equivalence was claimed between these values, as a difference in RNA/DNA of less than 0.5 log10 should not be taken into consideration due to the intra-assay standard deviation of molecular tests, which is estimated to be ±0.5 log10 unit (17, 19).
RESULTS
HCV RNA stability.
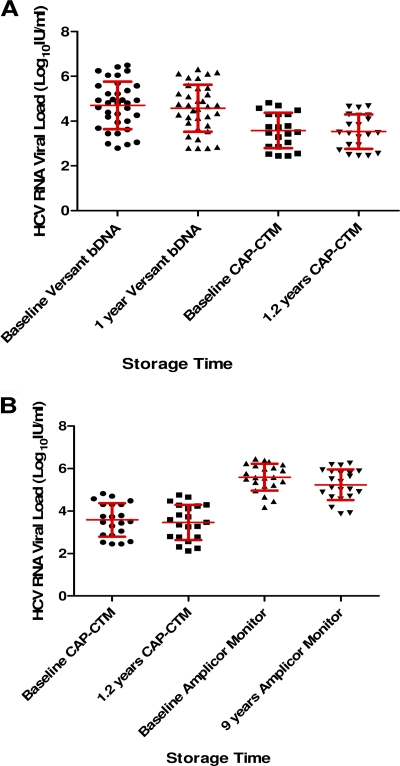
The results of the viral load testing of 56 HCV RNA samples stored for 1 to 1.2 years at −20°C are shown in Fig. 1A. There was a difference in the level of HCV RNA between samples tested at baseline and after storage at −20°C using the Versant HCV RNA 3.0 (bDNA) assay or CAP-CTM assay (means ± SDs, 0.13 ± 0.09 and 0.05 ± 0.15 IU/ml, respectively). There was also a decline in the mean viral load of 22 HCV RNA samples measured with the Cobas Amplicor Monitor assay at baseline and after storage for 9 years at −70°C (mean ± SD, 0.35 ± 0.19 IU/ml; Fig. 1B). Figure 2 shows that overall there was a decline in the mean viral load for HCV RNA-positive samples stored at −20°C and −70°C. The mean of the difference in viral loads within identical samples stored at −20°C and −70°C (see Materials and Methods) was statistically significant (P < 0.0001), indicating a greater loss of HCV viral titer following storage at −70°C. However, the standard deviations were greater than the mean RNA loss, indicating a significant intra-assay variation that can explain this difference. Overall the ±95% CI was less than 0.5 log10 IU/ml in all cases.
Fig. 1.
(A) Mean ± SD HCV viral load (log10 IU/ml) at baseline and after storage at −20°C tested using the Versant HCV RNA 3.0 (bDNA) assay and CAP-CTM assay; (B) mean ± SD HCV viral load (log10 IU/ml) at baseline and after storage at −70°C tested using the Cobas Amplicor HCV Monitor (version 2.0) assay (Monitor).
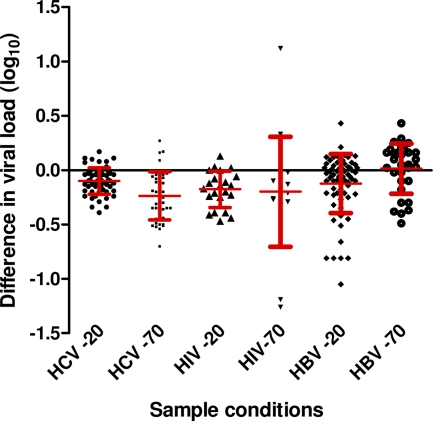
Fig. 2.
Mean difference in viral load (log10) ± standard deviation for HIV, HCV, and HBV after storage at −20°C and −70°C from 1 to 9.1 years.
HIV-1 RNA stability.
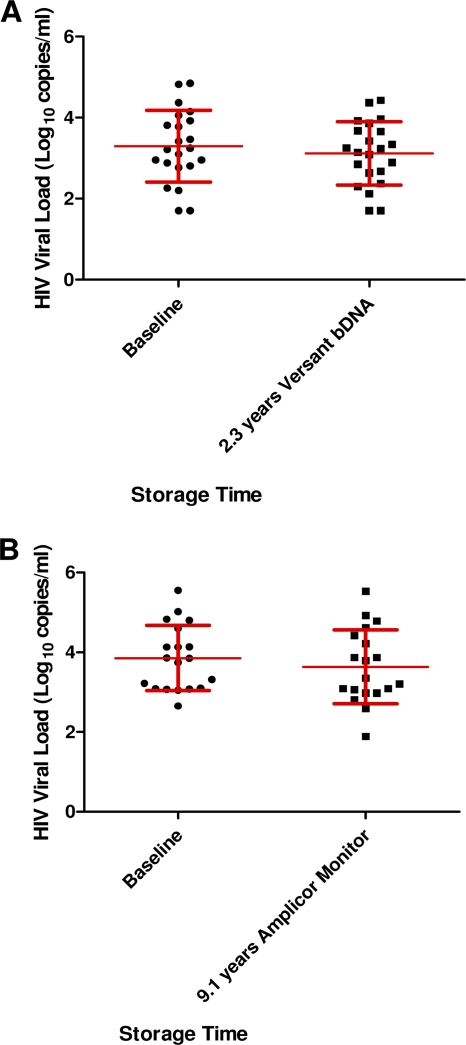
The mean viral load of 22 HIV-1 RNA samples measured with the Versant HCV RNA 3.0 (bDNA) assay at baseline and after storage at −20°C for 2.3 years showed RNA loss (mean ± SD, 0.18 ± 0.17; Fig. 3A). Figure 3B shows the results from 19 HIV-1 RNA samples following storage for up to 9.1 years at −70°C measured with the Cobas Amplicor Monitor assay. In three of the samples, a viral load loss of greater than 0.5 log10 copy/ml was observed. Samples measured with this assay also exhibited a SD of 0.51, indicating a significant intra-assay variation. Overall, there was no significant difference (P = 0.73) between the mean of the difference in viral loads following storage at −20°C and −70°C (Fig. 2), and the 95% CIs were less than 0.5 log10 IU/ml under both storage conditions.
Fig. 3.
(A) Mean ± SD HIV RNA load (log10 number of copies/ml) at baseline and after storage at −20°C for 2.3 years using the Versant HCV RNA 3.0 (bDNA) test; (B) mean ± SD HIV RNA load (log10 number of copies/ml) at baseline and after storage at −70°C for up to 9.1 years tested using the Cobas Amplicor HIV-1 Monitor (version 1.5) test.
HBV DNA stability.
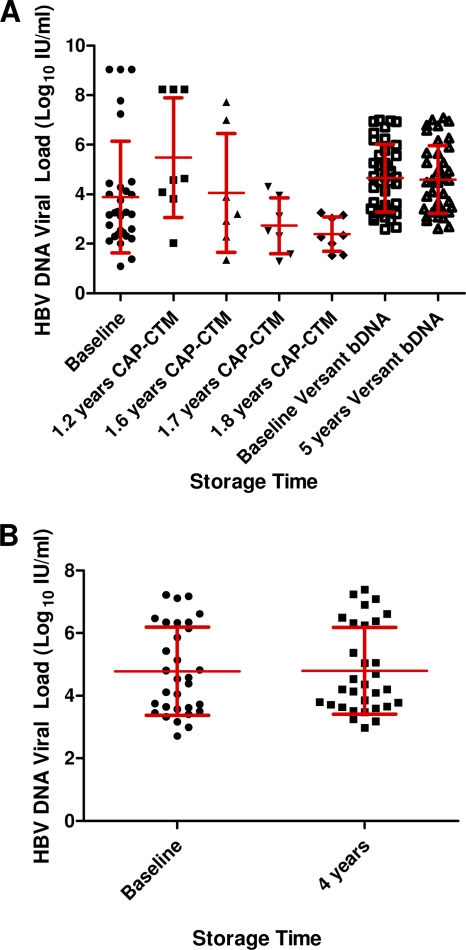
There was a significant decline in the mean HBV DNA load of four samples measured with the CAP-CTM assay before and after storage at −20°C for 1.8 years (Fig. 4 A). The mean HBV viral load showed a difference for 40 samples stored at −20°C for up to 5 years (mean ± SD, 0.04 ± 0.19; Fig. 2) and for 31 samples stored at −70°C for up to 4 years (mean ± SD, 0.01 ± 0.23) There was a statistically significant difference (P < 0.005) between the mean of the difference in viral loads following storage at −20°C and −70°C, indicating a slightly greater loss of HBV viral titer following storage at −20°C. As with previous results, the standard deviations were greater than the mean DNA loss before and after storage. Furthermore, the 95% CI was less than 0.5 log10 IU/ml in both cases (Fig. 4B).
Fig. 4.
(A) Mean ± SD HBV DNA load (log10 IU/ml) at baseline and after storage at −20°C for up to 5 years tested using the CAP-CTM assay and the Versant HCV RNA 3.0 (bDNA) test; (B) mean ± SD HBV DNA load (log10 IU/ml) at baseline and after storage at −70°C for up to 4 years tested using the Versant HCV RNA 3.0 (bDNA) test.
DISCUSSION
Quantitative viral NATs are important in assessing the response to antiviral therapy, as a surrogate for predicting clinical progress, and in some settings as a confirmatory diagnostic assay (2, 5, 16). It is imperative that blood collection, processing, and storage methods be optimized to ensure the accuracy and reproducibility of viral load test results. This should apply within a specific laboratory, among different laboratories, and across different test methodologies.
Most studies demonstrate no substantial loss of HIV-1 or HCV viral titer during storage over many years, although some studies show reductions with time (6, 13, 15, 19). Ginocchio et al. found significant decreases in the mean plasma HIV-1 load when the plasma was stored for over 6 months at −70°C, although these investigations used a lower acceptability range (0.3 log versus the 0.5 log used in this study) (10). Todd et al. found a significant decline in HIV RNA levels in samples stored at −20°C compared with those in samples stored at −80°C after 6 months of storage, where a 30 to 80% decrease in HIV-1 RNA levels was observed at −20°C compared to −80°C (20). In the current study, similar losses at −20°C and −70°C were reported following storage for up to 2.3 years and 9.1 years, respectively (Table 1).
Table 1.
Mean difference in viral loads, standard deviations, and 95% confidence intervals of HCV RNA, HIV RNA, and HBV DNA over 1 year to 9.1 years for specimens stored at −20°C and −70°C
| Virus | Time period (yr) | Assay | Storage temp (°C) | Difference in viral load (log10) |
95% CI |
||
|---|---|---|---|---|---|---|---|
| Mean | SD | Upper | Lower | ||||
| HCV | 1 | bDNA | −20 | −0.13 | 0.09 | −0.11 | −0.16 |
| 1.2 | CAP-CTM | −20 | −0.05 | 0.15 | 0.02 | −0.11 | |
| 1.2 | CAP-CTM | −70 | −0.11 | 0.18 | −0.03 | −0.19 | |
| 9 | Monitor | −70 | −0.35 | 0.19 | −0.27 | −0.43 | |
| HIV | 2.3 | bDNA | −20 | −0.18 | 0.17 | −0.11 | −0.25 |
| 9.1 | Monitor | −70 | −0.22 | 0.51 | −0.14 | −0.3 | |
| HBV | 1.8 | CAP-CTM | −20 | −0.21 | 0.37 | −0.16 | −0.25 |
| 5 | bDNA | −20 | −0.04 | 0.19 | −0.03 | −0.07 | |
| 4 | bDNA | −70 | 0.01 | 0.23 | 0.04 | −0.26 | |
Sebire et al. (19) showed that the HCV RNA titer was stable for 12 months at −70°C, which is consistent with the findings of Damen et al. (6), who demonstrated HCV RNA stability for at least 15 months at −70°C. Halfon et al. observed stability of HCV RNA for up to 6 months following storage at −80°C, with the loss of only 10% of the HCV titer, but reported a significant decrease (23%) following storage at −20°C (11). In contrast, José et al. showed no reduction in detectable HCV RNA and HIV RNA following storage at −20°C over periods of up to 5 years (13, 14). In the current study, a significantly greater loss (P < 0.0001) of HCV RNA viral load was seen after storage at −70°C than at −20°C (Table 1).
There are fewer published studies investigating the stability of HBV DNA under specific storage temperatures. Gessoni et al. studied the stability of samples containing different titers of HCV, HIV-1, and HBV. This study showed that while HCV and HIV-1 RNA can be stored for 72 h at 4°C, HBV DNA can be stored until 168 h (7 days) without lowering the viral titer (9). José et al. showed that HBV DNA stored at +5°C and +25°C was stable for at least 28 days, regardless of initial titer (14). The longer stability of HBV DNA than HCV and HIV RNA has been attributed to the principle that DNA is more stable than RNA and, hence, more resistant to the effects of storage conditions (7, 14).
The conflicting results may be explained by the fact that in some cases the coefficient of variation (CV) of the molecular assays is greater than the real titer decay (9, 11). The CV of the Versant HCV RNA 3.0 (bDNA) assay ranges from 14 to 26%, that of the CAP-CTM assay ranges from 15 to 30%, and that of the Cobas Amplicor Monitor assay ranges from 22 to 34.5%. This intra-assay variation inherent to molecular assays is shown in the high standard deviations of all viral load measurements, which in 6/9 cases exceeded the mean RNA/DNA loss (Table 1). Therefore, official recommendations have stated that HIV and HCV RNA titers be expressed in log10 and that variations of less than 0.5 log10 copies/ml (3 to 6 log10 units of the absolute number of genome copies by a factor of 3) not be considered significant (17).
The question arises: should we use classical statistics to test this type of variation? As we know, “statistically significant” simply means that the statistics used are reliable, but it does not mean that the finding is important, nor does it have a decision-making utility, because it can be weak or strong or large or small according to the method used, the null hypothesis, and the sample size. As mentioned above, it is generally accepted for molecular assays that a variation of less than 0.5 log10 RNA copy number/ml and 0.5 log10 RNA IU/ml should not be taken into account (2, 17, 19). The absolute values of HIV RNA loss at −20°C and −70°C (−0.18 and −0.22, respectively) are similar to those for HCV RNA (−0.13 and −0.35, respectively), yet Student's t test (2-tailed) brings forward a significant difference for HCV and a nonsignificant difference for HIV. We thus propose to take the two-sided 95% CI of the RNA/DNA difference before and after storage within ±0.5 log10 unit to be the acceptable limit of RNA loss.
While results showed a statistically significant loss (P < 0.005) of HCV RNA and HBV DNA following storage at −20°C and −70°C, the standard deviations were greater than the mean RNA/DNA loss in 60% (6/9) of the cases, indicating a significant intra-assay variability. Marked differences could have been found if the same sample had been tested on different occasions. Overall, the 95% CI was less than 0.5 log10 IU/ml in all cases, using different assays: the Cobas AmpliPrep/Cobas TaqMan, Cobas Amplicor Monitor, and Versant bDNA assays. Despite the different methodologies underlying these assays, there is a good correlation between the quantitation of HIV and HCV RNA and HBV DNA using the TaqMan, Monitor, and bDNA assays over a wide range of RNA/DNA levels (8, 22). The type of assay for RNA/DNA quantitation in the current study did not affect the overall viral load levels following storage.
In conclusion, using three different assays (Versant bDNA assays and Cobas Ampliprep/Cobas TaqMan and Cobas Amplicor Monitor assays), we have shown that samples stored at −70°C for up to 9 years exhibited a significant loss of HCV RNA load, consistent with the results of previous studies, although it was less than 0.5 log10 HCV RNA titer. Similarly, HBV DNA samples stored at −70°C for up to 4 years did show a significant loss of HBV viral load. The results also show a loss of less than 0.5 log10 HBV DNA, HCV RNA, and HIV-1 RNA at up to 5 years, 2 years, and 2.3 years of storage at −20°C, respectively. Such samples are adequate for the detection of HCV, HIV-1, and HBV nucleic acids using NATs for the assessment of the infectious risk of cell, blood, and tissue samples from donors.
ACKNOWLEDGMENTS
We thank Cornelia Escano (Virology Diagnostic Laboratory, SEALS Microbiology) for performing some of the tests on the Cobas AmpliPrep/Cobas TaqMan tests and Dominic Dwyer for provision of a subset of HIV samples.
Footnotes
Published ahead of print on 13 July 2011.
REFERENCES
- 1. Ballen K. K. 2005. New trends in umbilical cord blood transplantation. Blood 105:3786–3792 [DOI] [PubMed] [Google Scholar]
- 2. Best S. J., Gust A. P., Johnson E. I., McGavin C. H., Dax E. M. 2000. Quality of human immunodeficiency virus viral load testing in Australia. J. Clin. Microbiol. 38:4015–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brunstein C. G., Setubal D. C., Wagner J. E. 2007. Expanding the role of umbilical cord blood transplantation. Br. J. Haematol. 137:20–35 [DOI] [PubMed] [Google Scholar]
- 4. Busch M. P., Wilber J. C., Johnson P., Tobler L., Evans C. S. 1992. Impact of specimen handling and storage on detection of hepatitis C virus RNA. Transfusion 32:420–425 [DOI] [PubMed] [Google Scholar]
- 5. Chevaliez S. B., Bouvier-Alias M., Brillet R., Powlotsky J. M. 2007. Overestimation and underestimation of hepatitis C virus RNA levels in a widely used real-time PCR-based method. Hepatology 46:22–31 [DOI] [PubMed] [Google Scholar]
- 6. Damen M., et al. 1998. Stability of hepatitis C RNA during specimen handling and storage prior to NASBA amplification. J. Virol. Methods 72:175–184 [DOI] [PubMed] [Google Scholar]
- 7. Ehrlich G. D., Greenberg S. J. 1994. PCR-based diagnostics in infectious disease, vol. 2 Blackwell Scientific Publications, London, United Kingdom [Google Scholar]
- 8. Garbuglia A. R., et al. 2007. Comparison of Versant HBV DNA 3.0 and COBAS AmpliPrep-COBAS TaqMan assays for hepatitis B DNA quantitation: possible clinical implications. J. Virol. Methods 146:274–280 [DOI] [PubMed] [Google Scholar]
- 9. Gessoni G., et al. 2004. Biological qualification of blood units; considerations about the effects of sample's handling and storage on stability of nucleic acids. Trans. Apheresis Sci. 30:197–203 [DOI] [PubMed] [Google Scholar]
- 10. Ginocchio C., et al. 1997. Effects of specimen collection, processing, and storage conditions on stability of human immunodeficiency virus type 1 RNA levels in plasma. J. Clin. Microbiol. 35:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halfon P., et al. 1996. Impact of various handling and storage conditions on quantitative detection of hepatitis C virus RNA. J. Hepatol. 25:307–311 [DOI] [PubMed] [Google Scholar]
- 12. Jager M., et al. 2007. Bone healing and migration of cord blood-derived stem-cells into a critical size femoral defect after xenotransplantation. J. Bone Mineral Res. 22:1224–1233 [DOI] [PubMed] [Google Scholar]
- 13. José M. C. S., Gajardo R., Jorquera J. I. 2003. The effect of storage at different temperatures on the stability of hepatitis C virus RNA in plasma samples. Biologicals 31:1–8 [DOI] [PubMed] [Google Scholar]
- 14. José M., Gajardo R., Jorquera J. I. 2005. Stability of HCV, HIV-1 and HBV nucleic acids in plasma samples under long-term storage. Biologicals 33:9–16 [DOI] [PubMed] [Google Scholar]
- 15. Lew J., et al. 1998. Determinations of levels of human immunodeficiency virus type 1 RNA in plasma: reassessment of parameters affecting assay outcome. J. Clin. Microbiol. 36:1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nowak M. A., et al. 1996. Viral dynamics in hepatitis B virus infection. Proc. Natl. Acad. Sci. U. S. A. 93:4398–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pawlotsky J. M. 1997. Measuring hepatitis C viremia in clinical samples: can we trust the tests? Hepatology 26:1–4 [DOI] [PubMed] [Google Scholar]
- 18. Saldanha J. 2001. Validation and standardisation of nucleic acid amplification technology (NAT) assays for the detection of viral contamination of blood and blood products. J. Clin. Virol. 20:7–13 [DOI] [PubMed] [Google Scholar]
- 19. Sebire K., McGavin K., Land S., Middleton T., Birch C. 1998. Stability of human immunodeficiency virus RNA in blood specimens as measured by a commercial PCR-based assay. J. Clin. Microbiol. 36:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Todd J., et al. 1995. Performance characteristics for the quantitation of plasma HIV-1 RNA using branched DNA signal amplification technology. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:35–44 [PubMed] [Google Scholar]
- 21. Yao F., et al. 2007. The risk of HIV, HBV, HCV and HTLV infection among musculoskeletal tissue donors in Australia. Am. J. Transplant. 7:2723–2726 [DOI] [PubMed] [Google Scholar]
- 22. Yao J. D. C., et al. 2004. Multicenter evaluation of the VERSANT hepatitis B virus DNA 3.0 assay. J. Clin. Microbiol. 42:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]