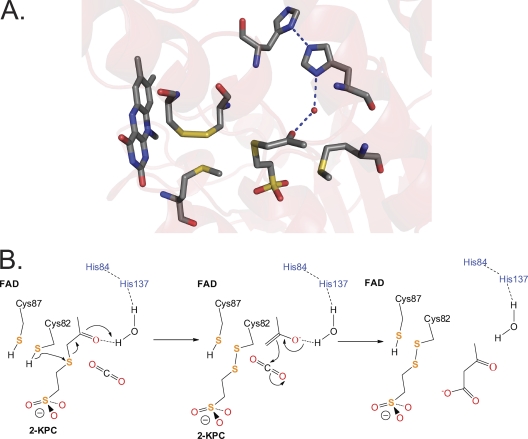
Fig. 3.
Active-site architecture and proposed mechanism for 2-KPCC. (A) Structure of 2-KPC bound to 2-KPCC, highlighting active-site residues believed to be key to catalysis (Protein Data Bank ID, 1MO9). (B) Proposed mechanism of thioether bond cleavage, enolacetone formation and stabilization, and carboxylation based on the structures solved for 2-KPCC and the results of the present work. The initial abstraction of a proton from C82 may be facilitated by a general base that has not yet been identified. The reduction of the mixed disulfide of CoM and Cys82 is not shown but will occur as for glutathione reductase (Fig. 2B).