Abstract
Cupriavidus metallidurans is adapted to high concentrations of transition metal cations and is a model system for studying metal homeostasis in difficult environments. The elemental composition of C. metallidurans cells cultivated under various conditions was determined, revealing the ability of the bacterium to shield homeostasis of one essential metal from the toxic action of another. The contribution of metal uptake systems to this ability was studied. C. metallidurans contains three CorA members of the metal inorganic transport (MIT) protein family of putative magnesium uptake systems, ZupT of the ZRT/IRT protein, or ZIP, family, and PitA, which imports metal phosphate complexes. Expression of the genes for all these transporters was regulated by zinc availability, as shown by reporter gene fusions. While expression of zupT was upregulated under conditions of zinc starvation, expression of the other genes was downregulated at high zinc concentrations. Only corA1 expression was influenced by magnesium starvation. Deletion mutants were constructed to characterize the contribution of each system to transition metal import. This identified ZupT as the main zinc uptake system under conditions of low zinc availability, CorA1 as the main secondary magnesium uptake system, and CorA2 and CorA3 as backup systems for metal cation import. PitA may function as a cation-phosphate uptake system, the main supplier of divalent metal cations and phosphate in phosphate-rich environments. Thus, metal homeostasis in C. metallidurans is achieved by highly redundant metal uptake systems, which have only minimal cation selectivity and are in combination with efflux systems that “worry later” about surplus cations.
INTRODUCTION
Sophisticated cellular biochemistry needs metals as cofactors. About 40% of all enzymes have them, ranking from Mg (16%) > Zn (9%) > Fe (8%) > Mn (6%) > Ca (2%) > Co and Cu (1%) down to K, Na, Ni, V, Mo, W, and only one example of Cd (59). It is an interesting question how the correct metal is allocated to the right protein, a challenge especially for the divalent metal cations Mg2+, Zn2+, Fe2+, Mn2+, Co2+, Ni2+, and Cu2+. These metals compete with each other for the metal binding sites in enzymes (16). Additionally, Fe2+/3+ and Cu+/2+ promote dangerous reactive oxygen species in Fenton and Fenton-like reactions, as described by Haber and Weiss (13).
Part of the solution to this problem might be to keep the metal cation bouquet in any cellular compartment in a way that minimizes competition for metal binding sites and the Fenton reaction. This leads to the question of how the cellular metal cation bouquet can be maintained in environments that may contain a single metal in a concentration range from pM to mM. The betaproteobacterium Cupriavidus metallidurans strain CH34 is able to keep its metal homeostasis under a variety of such adverse conditions (19, 28, 30). The organism can be found in many mesophilic metal-contaminated environments around the globe, such as zinc deserts of Belgium (8). Key to this adaptive ability is an impressive array of metal efflux systems (highlighted for zinc in Table 1) (57).
Table 1.
Inventory of possible and absent zinc transport systems in C. metallidurans strain CH34
| Name | Rmet no.a | Transporter familyb | Possible functionc | Reference(s) |
|---|---|---|---|---|
| Uptake systems | ||||
| CorA1 | Rmet_3052 | MIT, TC 1.A.35 | Importer for divalent metal cations; Mg2+ regulation | |
| CorA2 | Rmet_0036 | MIT, TC 1.A.35 | Importer for divalent metal cations; no Mg2+ regulation | |
| CorA3 | Rmet_3287 | MIT, TC 1.A.35 | Importer for divalent metal cations; no Mg2+ regulation | |
| ZupT | Rmet_2621 | ZIP, TC 2.A.5 | Uptake of Zn2+ and other cations under conditions of low zinc concentrations | |
| HoxN | Rmet_1533 | NiCoT, TC 2.A.52 | Ni2+, Co2+, synthesis of hydrogenases. | |
| PitA | Rmet_1973 | PiT, TC 2.A.20 | Phosphate-metal cation complexes: Zn2+, Co2+, Ni2+, Cd2+ | |
| MgtE | Absent | MgtE, TC 9.A.19 | Possible substrates Mg2+, Co2+ | |
| MntH | Absent | NRAMP, TC 2.A.55 | Possible substrates Mn2+, Fe2+, Cd2+, Co2+, Ni2+ | |
| ZnuABC | Absent | ABC, TC 3.A.1 | High-affinity uptake of zinc under conditions of zinc starvation | |
| MgtA | ? | P type, TC 3.A.3 | Primary uptake system for Mg2+ and other divalent cations | |
| Efflux systems | ||||
| CzcA | Rmet_5980 | RND, TC 2.A.6 | Together with CzcB and CzcC, outer membrane efflux of Zn2+; other substrates Cd2+ and Co2+ | 10, 39, 51 |
| CzcP | Rmet_5970d | P-type, TC#3.A.3 | High Vmax efflux of loosely bound Zn2+; other substrates Cd2+ and Co2+ | 51 |
| ZntA | Rmet_4594 | P type, TC 3.A.3 | Efflux of tightly bound Zn2+; other substrate Cd2+ | 24, 51 |
| PbrA | Rmet_5947 | P type, TC 3.A.3 | Efflux of tightly bound Pb2+; may use Zn2+ and Cd2+ as substrates | 7, 51 |
| CadA | Rmet_2303d | P type, TC 3.A.3 | Efflux of tightly bound Cd2+; may use Zn2+ as substrate | 24, 51 |
| CzcD | Rmet_5979 | CDF, TC 2.A.4 | Efflux of loosely bound Zn2+, other substrates Cd2+ and Co2+ (2, 3, 36, 51) | |
| DmeF | Rmet_0198 | CDF, TC 2.A.4 | Efflux of Co2+; Zn2+ not substrate in vivo | 32, 51 |
| FieF | Rmet_3406 | CDF, TC#2.A.4 | Efflux of Fe2+; Zn2+ not substrate in vivo | 32, 51 |
| Unclear | ||||
| ZntB | Rmet_0549 | MIT, TC 1.A.35 | Efflux or uptake of Zn2+? |
Because the cytoplasmic concentration of each metal may be based on a kinetic flow equilibrium of uptake and efflux reactions (see Fig. S1 in the supplemental material), real understanding of multiple-metal homeostasis also requires knowledge of the uptake systems. However, little is known about the import of these cations into C. metallidurans. The divalent metal cations of zinc, cadmium, cobalt, nickel, and manganese are transported into cells of the plasmid-free C. metallidurans derivative AE104 by energy-dependent magnesium transport systems with Km values in the mid-μM range (41). Careful analysis of the uptake data (25) revealed the presence of at least two uptake systems for transition metal cations. A slow (low maximum rate of transport [Vmax]) system functions mainly at low (≤100 μM) cation concentrations and a faster one at concentrations above this value. The slow uptake system displayed kinetic parameters similar to those of the previously published magnesium transport system(s). However, the molecular identities of all these uptake systems remain unknown, although the bacterium contains a unique repertoire of secondary transport systems that might be responsible for the observed transport processes (Table 1). Secondary transport systems are driven by a gradient across a biological membrane, such as the proton motive force, while primary systems form gradients by using other forms of chemical energy (e.g., ATP or PEP), redox differences, or light (49).
C. metallidurans does not contain a high-affinity Zn2+ uptake system like the ABC (TC 3.A.1) uptake system ZnuABC from Escherichia coli (44) but an ortholog of ZupT (23) of the ZIP protein family (TC 1.A.35). Reminiscent of the situation with a multitude of paralogous efflux systems (57), C. metallidurans contains 4 members of the CorA or metal inorganic transport (MIT) (TC 1.A.35) protein family. The three CorA1 to CorA3 proteins exhibit a conserved “GMNFXXMPEL” sequence motif, while only the first “GMN” and the proline residue are conserved in the remaining MIT protein, ZntB. While CorA from Salmonella is not involved in zinc import (54), ZntB could be a zinc efflux protein (62). The structure of ZntB is different from that of CorA, and ZntB contains three zinc-binding sites, two of them probably required for zinc transport (60).
An NRAMP (TC 2.A.55) protein similar to E. coli MntH, which imports Mn2+ but also Fe2+ and the toxic Cd2+, is missing in C. metallidurans (Table 1). Since a SitABC-type uptake system for manganese/iron and a housekeeping manganese-dependent superoxide dismutase are also absent, strain CH34 may have little use for manganese. Alternatively, zinc and other metals could be imported by PitA, which may transport phosphate-metal cation complexes at high transition metal concentrations, as in E. coli (6). Thus, PitA could be responsible for the observed fast uptake of metals at high (>100 μM) concentration (25). Finally, HoxN could be a slow nickel uptake system required to provide the metal for hydrogenase biosynthesis (9). However, the HoxN from C. metallidurans is not an ortholog of the HoxN from Cupriavidus eutrophus because it belongs to a different subfamily of NiCoT proteins (57).
To understand the contribution of uptake systems to the adjustment of the cellular metal bouquet in C. metallidurans, first, the metal bouquet itself was quantified, and how C. metallidurans maintains it under various conditions was investigated. Interestingly, the bacterium is able to shield homeostasis of all other metals when one metal is present at high concentrations, and it is able to handle high concentrations of many metals in parallel. The question was how this was achieved. The efflux systems of C. metallidurans each may be assigned to a central substrate cation (51), e.g., DmeF to cobalt, CadA to cadmium, ZntA to zinc, PbrA to lead, FieF to iron, and CnrT to nickel (see Fig. S1 in the supplemental material). How do the uptake systems fit into this picture? Do a specific uptake system and a specific efflux system, both controlled by their central substrate, line up to form a “shunt” (see Fig. S1 in the supplemental material), such as ZnuABC and ZntA in E. coli (43), or do the uptake systems import more or less what they can get and leave it to the efflux systems to “worry later” about any surplus cation?
To keep our approach manageable, here, we ignored all primary uptake systems, the putative nickel importer HoxN, and the possible efflux system ZntB. Moreover, to circumvent interference with the metal resistance determinants on the native plasmids pMOL28 and pMOL30 of C. metallidurans, mutants in metal uptake systems were constructed in the megaplasmid-free strain AE104, which was also used in the first physiological characterization of metal import (41). The studied systems ZupT, PitA, and CorA1, CorA2, and CorA3 were all controlled by the environmental zinc concentration (and magnesium in the case of CorA1), and functions in metal homeostasis could be assigned to these importers.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used for experiments were E. coli strain W3110 (5), C. metallidurans strain CH34(pMOL28, pMOL30) wild type, and derivatives of the megaplasmid-free strain AE104 (29). Tris-buffered mineral salts medium (29) containing 2 g sodium gluconate/liter (TMM) was used to cultivate these strains aerobically with shaking at 30°C. Analytical-grade salts of heavy metal chlorides were used to prepare 1 M stock solutions, which were sterilized by filtration. Solid Tris-buffered media contained 20 g agar/liter (Difco). If not otherwise indicated, TMM contained trace element solution SL6 (45), which added the following final concentrations to the TMM: 35 nM ZnCl2, 15 nM MnCl2, 84 nM CoCl2, 5.9 nM CuCl2, 8.4 nM NiCl2, 12 nM Na2MoO4, and 485 nM H3BO3.
Genetic techniques.
Standard molecular genetic techniques were used (34, 50). For conjugative gene transfer, overnight cultures of the donor strain E. coli S17/1 (53) and of the C. metallidurans recipient strains grown at 30°C in Tris-buffered medium were mixed (1:1) and plated onto nutrient broth agar. After 2 days, the bacteria were suspended in TMM, diluted, and plated onto selective media as previously described (34).
All primer pairs used are listed in Table S1 in the supplemental material. Plasmid pECD1003 was used to construct deletion mutants. It is a derivate of plasmid pCM184 (27). These plasmids harbor a kanamycin resistance cassette flanked by loxP recognition sites. Plasmid pECD1003 additionally carries alterations of 5 bp at each loxP site. Using these mutant lox sequences, multiple gene deletions within the same genome are possible without interference by secondary recombination events (1, 55).
Deletion mutants.
Fragments of 300 bp upstream and downstream of the target gene were amplified by PCR, cloned into the vector pGEM-T Easy (Promega), sequenced, and further cloned into the plasmid pECD889 or pECD1003. The resulting plasmids were used in a double-crossover recombination in C. metallidurans strains to replace the respective target gene by the kanamycin resistance cassette, which was subsequently also deleted by transient introduction of the cre expression plasmid pCM157 (27). Cre recombinase is a site-specific recombinase from the phage P1 that catalyzes the in vivo excision of the kanamycin resistance cassette at the loxP recognition sites. The correct deletions of the respective transporter genes were verified by Southern DNA-DNA hybridization. For construction of multiple-deletion strains, these steps were repeated. The resulting mutants carried a small open reading frame instead of the wild-type gene to prevent polar effects.
Gene insertions.
Although the corA1 gene could be interrupted by a single-crossover experiment as described above, recombinant strains containing a corA1 deletion as a consequence of a double-crossover event could never be isolated at this stage. Therefore, the corA1 gene had to be inactivated by insertional mutagenesis. Its central part was amplified by PCR from total DNA of strain AE104, cloned as a PstI/XbaI fragment into the plasmid pGEM-T easy (Promega), verified by DNA sequence analysis, and cloned into plasmid pECD794 (pLO2-lacZ) (26). For reporter operon fusions, lacZ was inserted downstream of several targets. This was done without interrupting any open reading frame downstream of the target genes to prevent polar effects. The 300- to 400-bp 3′ ends of the respective target genes were amplified by PCR from total DNA of strain AE104, and the resulting fragments were cloned into plasmid pECD794 (pLO2-lacZ). The respective operon fusion cassettes were inserted into the open reading frame of the target gene by single-crossover recombination.
Induction experiments.
C. metallidurans cells with a lacZ reporter gene fusion were cultivated in TMM with shaking at 30°C. At a cell density of 60 to 70 Klett units, heavy metal salts were added to various final concentrations and the cells were incubated with shaking for an additional 3 h or 16 h. The specific β-galactosidase activity was determined in permeabilized cells as published previously, with 1 U defined as the activity forming 1 nmol of o-nitrophenol per min at 30°C (36).
Transport assays.
65Zn2+ uptake experiments using the filtration method were performed as described previously (42) with some modifications. Cation uptake was started by the addition of the radioactive 65Zn2+ (NEN; 80 GBq/g). Samples (300 μl) were filtered through membrane filters (pore size, 0.45 μm; Schleicher and Schuell) and rinsed with 10 ml 10 mM Tris-HCl (pH 7.0) buffer containing 10 mM MgCl2. The radioactivity remaining on the membrane filter was determined with a scintillation counter (LS6500; Beckman).
ICP-MS analysis.
To determine the metal content, cells were cultivated in TMM to the end of the exponential phase of growth, and 1.5 ml cell suspension was centrifuged for 5 min at 4,500 × g. The supernatant was discarded, and the residual liquid was carefully removed with a sterile cotton swab. Additional washing of the cells did not improve the reproducibility of the method. The pellet was suspended in 20 μl concentrated 70% (wt/vol) HNO3 (trace metal grade; Mallinckrodt) and mineralized at 70°C for 3 h. Samples were diluted to a final concentration of 5% (wt/vol) nitric acid. Gallium [as Ga(NO3)3] was added as an internal standard at a final concentration of 50 ppb. Elemental analysis was performed via inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent ICP-MS 7500cx instrument operating with a collision cell and flow rates of 3.5 ml min−1 of H2 and 1.5 ml min−1 of He, with an Ar carrier flow rate of 0.95 liter min−1 and an Ar makeup flow rate of 0.15 liter min−1. Data acquisition for each sample was accumulated in triplicate for 100 milliseconds. An external calibration curve was recorded with gallium in 5% (wt/vol) nitric acid. Samples were loaded onto 96-well plates prior to analysis, and an autosampler (Elemental Scientific Inc.) was used to inject samples via a 6-port valve at a flow of 70 μl min−1. The results were transformed from ppm, ppb, or ppt via molar units into atoms per sample and divided by the number of cells per sample, which had been determined before as CFU.
RESULTS
Elemental composition of C. metallidurans.
To understand the adjustment of the metal bouquet in C. metallidurans, the elemental composition of C. metallidurans CH34 wild-type cells was determined and compared to that of the megaplasmid-free strain AE104 and that of E. coli W3110 as a “reference bacterium” (Table 2). Cells of strain CH34 contained the divalent metal cations in the order Mg ≫ Ca > Fe ≫ Zn > Cu > Ni ≫ Co ≫ Mn > Cd, indicating that iron and zinc are the most important transition metals (Table 2). Cadmium may have originated from contamination of the water or chemicals used. E. coli contained half as much iron but twice as much calcium and copper as C. metallidurans CH34. The biggest difference between the bacteria, however, was a nearly 12-fold-higher content of manganese in E. coli than in strain CH34, matching the absence of an NRAMP manganese uptake system and manganese-dependent superoxide dismutase (Table 1) in CH34.
Table 2.
Elemental compositions of C. metallidurans and mutant strains in comparison to that of E. coli strain W3110
| Strain or mutant | Composition (%)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg | P | Ca | Fe | Co | Ni | Cu | Zn | Mn | Cd | |
| E. coli | ||||||||||
| W3110 | 93 ± 3 | 70 ± 2 | 234 ± 38 | 54 ± 19 | 113 ± 47 | 90 ± 88 | 279 ± 167 | 125 ± 34 | 1,174 ± 159 | 87 ± 83 |
| C. metallidurans | ||||||||||
| AE104 | 90 ± 4 | 92 ± 1 | 96 ± 11 | 80 ± 9 | 189 ± 116 | 48 ± 9 | 59 ± 23 | 95 ± 6 | 83 ± 26 | 84 ± 34 |
| ΔzntA ΔcadA | 94 ± 6 | 94 ± 3 | 96 ± 1 | 90 ± 2 | 91 ± 1 | 38 ± 2 | 46 ± 4 | 104 ± 1 | 79 ± 7 | 150 ± 69 |
| ΔzupT | 104 ± 6 | 103 ± 7 | 131 ± 35 | 118 ± 16 | 362 ± 211 | 116 ± 40 | 92 ± 47 | 104 ± 5 | 122 ± 57 | 100 ± 35 |
| ΔzupT ΔpitA | 305 ± 24 | 179 ± 8 | 109 ± 5 | 90 ± 5 | 264 ± 127 | 87 ± 10 | 49 ± 10 | 82 ± 9 | 111 ± 16 | 85 ± 66 |
The elemental composition of C. metallidurans strain CH34, cultivated in Tris-buffered mineral salts medium, was determined by ICP-MS and calculated as atoms per cell as follows: Mg, 13.7 × 106 ± 0.8 × 106; P, 110 × 106 ± 3 × 106; Ca, 875 × 103 ± 165 × 103; Fe, 537 × 103 ± 103 × 103; Co, 3.97 × 103 ± 1.31 × 103; Ni, 10.8 × 103 ± 3.8 × 103; Cu, 60.7 × 103 ± 22.1 × 103; Zn, 90.9 × 103 ± 9.5 × 103; Mn, 993 × 100 ± 123 × 100; and Cd, 221 × 100 ± 254 × 100. Other elements were as follows: B, 27 ± 14 × 103; Na, 96.2 × 106 ± 45.3 × 106; S, 38.8 × 106 ± 13.8 × 106; K, 33.5 × 106 ± 8.0 × 106; Se, 13 × 106 ± 5 × 106; and Mo, 3.45 ± 0 × 103. Assuming a total cellular volume of 0.57 fl (11), these values would calculate to the following quotas (43) in decreasing order: 320 mM P, 280 mM Na, 110 mM S, 98 mM K, 40 mM Mg, 26 mM Ca, 16 mM Fe, 260 μM Zn, 180 μM Cu, 80 μM B, 31 μM Ni, 12 μM Co, 10 μM Mo, 2.9 μM Mn, 640 nM Cd, and 39 nM Se. The contents of the other C. metallidurans strains and that of E. coli strain W3110 are given as percentages of the C. metallidurans strain CH34 value. Interesting values are in boldface.
Megaplasmid-free C. metallidurans strain AE104 (29) accumulated half as much nickel and copper but twice as much cobalt as strain CH34; the latter value, however, had a large deviation (Table 2). Deletion of the genes for the P-type ATPases ZntA and CadA from the chromosome of strain AE104 did not change most metal contents under these conditions. Only the cobalt content decreased to the CH34 wild-type level, and the cadmium content was slightly elevated (Table 2). Thus, deletion of the two efflux P-type ATPases ZntA and CadA or of the metal resistance plasmids pMOL28 and pMOL30 did not disturb metal homeostasis at ambient metal concentrations.
Accumulation of metals under conditions of metal stress.
In the second step, the consequences of increasing concentrations of a metal for the cellular contents of the same metal and of other metals was examined in the three strains CH34, AE104, and E. coli W3110. Cell-bound metal increased when a particular metal was added to the growth medium (see Table S2 in the supplemental material). Nonamended TMM contained these metals in nM concentrations from trace element solution SL6 (45). Despite the more than 1,000-fold-higher concentrations, the total content of the added metal of the cells increased less than 20-fold under some bacterial strain/metal combinations: up to 100 μM Zn2+ for E. coli and strain AE104 and up to 100 μM Cu2+ or 30 μM Ni2+ for all three strains. Under most conditions, the cellular metal content was a linear function of the outside concentration (see Table S2 in the supplemental material, shaded areas), and the strains did not differ much in the total amount of metal bound to the cells. This indicated that binding of metals by the cells may have masked a possible adjustment of the periplasmic or cytoplasmic metal concentration by transport mechanisms.
Under three conditions (see Table S2 in the supplemental material, boxed areas), the cellular metal content increased more than the outside concentration: in E. coli and when Co2+ was ≥30 μM or Cd2+ was ≥10 μM. In strain CH34, the zinc content also increased strongly (see Table S2 in the supplemental material). Interestingly, increasing the content of one metal 1,000- or even 10,000-fold above the concentration in the TMM growth medium did not disturb the homeostasis of all the other metals (see Table S2 in the supplemental material). Most exceptions were observed when the cells approached the limits of their metal tolerances. In the case of CH34 and zinc, some coprecipitation with the zinc-binding process may have occurred. Otherwise, none of the three strains showed any disturbance of their ability to control homeostasis of one metal in the presence of another. To test the limits of the metal homeostasis system of strain CH34, the five cations Co2+, Ni2+, Cu2+, Zn2+, and Cd2+ were provided in a 1:1:1:1:1 ratio, and the metal content of CH34 cells was compared to that of the same metal under conditions when only a single metal was added (see Table S3 in the supplemental material). Up to 30 μM, the total content of each metal was about the same as if only a single metal had been added to the culture, and no disturbance of the homeostasis of another metal was measured. At 100 μM, however, all metal contents increased, and at 300 μM, growth stopped.
How may C. metallidurans handle high concentrations of multiple metals in parallel? Is it by a “shunt” of an uptake system for a single metal lined up with an efflux system for the same metal, with both controlled by the metal (see Fig. S1 in the supplemental material)? Or do the uptake systems take up what they can get and leave it to the efflux systems to “worry later” about any surplus metals?
Regulation of the expression of the genes for putative secondary metal uptake systems.
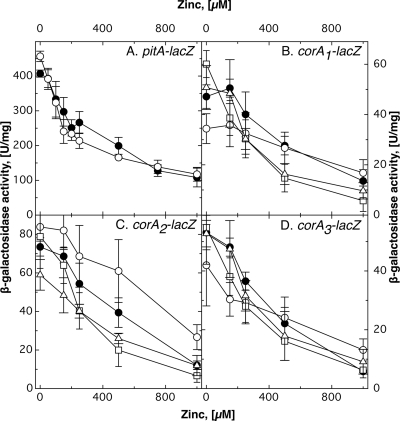
To discriminate between these possibilities, lacZ reporter gene fusions of the genes corA1, corA2, corA3, pitA, and zupT were constructed as operon fusions, leaving the gene of the importer intact, and regulation of these fusions by transition metals was studied. Expression of all five genes was downregulated with increasing zinc concentrations in the order zupT-lacZ (4.8-fold) > pitA-lacZ (3.6-fold) > corA3-lacZ (3.3-fold) > corA2-lacZ (3.0-fold) > corA1-lacZ (1.5-fold) (Fig. 1 and 2). The four other cations (Ni2+, Co2+, Cu2+, and Cd2+) had no effect on the expression of these genes, with the exception of corA1, which was slightly (1.4-fold) downregulated by either Cu2+ or Cd2+ (data not shown). This indicated that zinc availability was a major control element of transition metal homeostasis in C. metallidurans.
Fig. 1.
Regulation of expression of lacZ reporter gene fusions by zinc in mutant strains. Cells of C. metallidurans AE104 (•) mutant strains were incubated in TMM containing various concentrations of Zn2+. These strains carried lacZ fusions of pitA (A), corA1 (B), corA2 (C), or corA3 (D). The mutant strains were a ΔzupT deletion strain (○) (A to D) and a ΔpitA (□) and a ΔpitA ΔzupT (▵) deletion strain (B, C, and D). At least three independent determinations were made. The error bars indicate standard deviations.
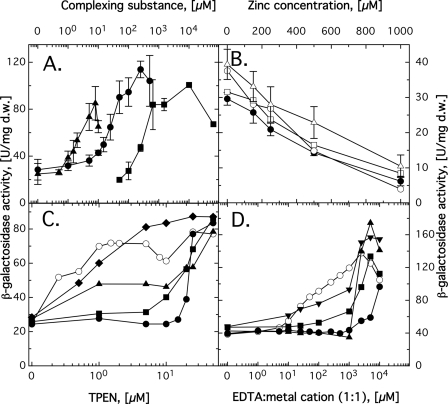
Fig. 2.
Regulation of expression of a zupT-lacZ reporter gene fusion. Cells of C. metallidurans AE104 carrying a zupT-lacZ fusion were incubated in TMM containing various concentrations of the chelating substance DPTA (▴), EDTA (•), or phosphate (▪) (A); zinc in the AE104 parent (•) or the ΔpitA (○), ΔcorA2 (□), or ΔcorA3 (▵) mutant (B); increasing concentrations of the chelating substance TPEN in the presence of 20 μM Fe3+ (♦), Mn2+ (▴), Co2+ (▪), or Zn2+ (•) or without added metal cations (○) (C); or increasing concentrations of EDTA-metal complexes in a 1:1 ratio, i.e., no metal (○), Zn2+ (•), Co2+ (▪), Mn2+ (▴), or Ni2+ (▾) (D). Incubation was continued for 16 h (phosphate) or 3 h (all others), and the β-galactosidase activity was determined. At least three independent determinations were made. Error bars indicating standard deviations are shown in panels A and B, but not in panels C and D to avoid cluttering. d.w., dry mass. Note that only panel B has a linear x axis.
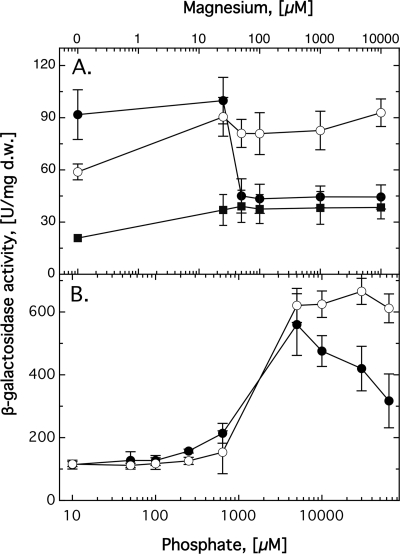
CorA1 production is downregulated by magnesium.
The corA1 gene, encoding the MIT transporter with the highest similarity to CorA from E. coli (data not shown), was the only gene that was upregulated with decreasing magnesium concentrations (Fig. 3A). Expression of the other four genes was not at all influenced by the Mg2+ concentrations when the trace element solution SL6 (containing 35 nM ZnCl2 in addition to other transition metal salts) was added to the TMM (shown in Fig. 3 for corA2-lacZ and corA3-lacZ; other data not shown). In the absence of SL6, the magnesium concentration affected the expression of these genes less than 2-fold (data not shown). Regulation of corA1-lacZ was not influenced by SL6 (data not shown). This indicated that CorA1 may be the main secondary uptake system for Mg2+ in C. metallidurans.
Fig. 3.
Regulation of expression of pitA and the corA proteins. (A) Influence of increasing Mg2+ concentrations on the expression of a corA1-lacZ (•), a corA2-lacZ (○), and a corA3-lacZ (▪) fusion. (B) Influence of increasing phosphate concentrations on the expression of a pitA-lacZ fusion (with pitA intact) in a parental background (•) and in a ΔzupT deletion strain (○). Precultures were washed twice and used to inoculate the main cultures, which were incubated for 16 h with shaking at 30°C in TMM containing SL6. Following incubation, β-galactosidase activity was determined. Note the log scale of the x axis in panel B. Five to seven independent determinations were made. The error bars indicate standard deviations. d.w., dry mass.
PitA production is upregulated by phosphate.
On the other hand, all transport systems except corA1 were upregulated at high phosphate concentrations, although the effect of increasing phosphate concentrations on corA2-lacZ or corA3-lacZ activity was below 2-fold (Fig. 2A [for zupT-lacZ] and data not shown). Since phosphate may complex with substrate cations, such as Zn2+ [solubility constant of Zn3(PO4)2 complex, 9 × 10−33 (61)], this upregulation may also have been due to complexation of zinc by phosphate, which decreased zinc availability to the cells.
The phosphate uptake system PitA was about 5-fold upregulated at a phosphate concentration of 5 mM. The expression level declined again at higher phosphate concentrations (Fig. 3B). This was unusual, since expression of the gene of an uptake system should be downregulated by increasing substrate concentrations, indicating that the focus of the cellular action of PitA may be more the cation uptake than that of phosphate.
Only zupT expression was upregulated by transition metal starvation.
Expression of zupT-lacZ was strongly upregulated, not only by EDTA and phosphate, but also by DPTA (diethylenetriaminepentaacetate) and TPEN [N,N,N′,N-tetrakis(2-pyridylmethyl) ethylenediamine] (Fig. 2). In contrast, none of the genes corA1i, corA2, corA3, and pitA was influenced by the EDTA concentration (data not shown). This indicates that zupT was the only gene for a secondary uptake system studied that was upregulated by starvation for divalent metal cations.
The influence of EDTA and TPEN on zupT-lacZ expression was more closely examined. Upregulation of zupT-lacZ expression by TPEN (Fig. 2C) was retarded in the presence of zinc, cobalt, and manganese (the effects were in the order Zn2+ > Co2+ > Mn2+ [Fig. 2C]), while the effect of Fe3+ was negligible. A concentration of 50 μM EDTA yielded 2.4- ± 0.5-fold upregulation. Addition of equimolar amounts of metal cations decreased this upregulation (Fig. 2D). Again, Zn2+ had the strongest effect; Ni2+, Co2+, Cd2+, Cu2+, and Mn2+ had some effect (shown for Ni2+, Co2+, and Mn2+ in Fig. 2D; Cd2+ not shown but similar to Co2+; Cu2+ not shown but similar to Mn2+); and Fe3+, Ca2+, and Mg2+ had no effect (data not shown).
Taken together, the environmental zinc concentrations, but not those of other metals, regulated the expression of all five genes. Additionally, zinc starvation regulates the expression of zupT. Moreover, corA1 was upregulated by magnesium starvation. Thus, no evidence for “shunts” was obtained.
Effect of zupT deletion on metal homeostasis.
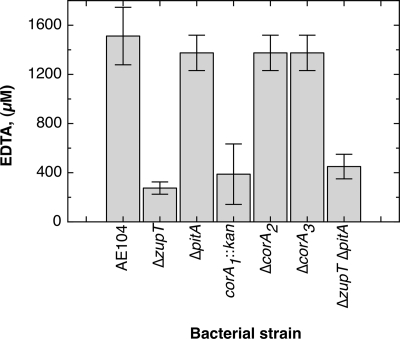
Deletion of zupT did not change the metal resistance of C. metallidurans strain AE104 significantly (see Table S4 in the supplemental material). After cultivation in Tris-buffered mineral salts medium, the metal content of ΔzupT cells was similar to that of strain AE104 with the exception of increased cobalt content (Table 2). ZupT was therefore not the only uptake system for any metal. However, ΔzupT led to a strong decrease of EDTA resistance on solid growth medium from a MIC of 1.5 mM to about 300 μM (Fig. 4). Addition of zinc more than that of cobalt abolished EDTA sensitivity (0.5 mol metal/mol EDTA; see Table S5 in the supplemental material). This indicated that ZupT was required to obtain zinc and possibly also cobalt under conditions of low cation availability.
Fig. 4.
MICs of various C. metallidurans mutant strains. The strains were grown for 48 h in TMM, diluted 1:100, and streaked on TMM agar plates with increasing EDTA concentrations. Growth was monitored after 5 days at 30°C. The results were confirmed by at least three independent experiments. The error bars indicate standard deviations.
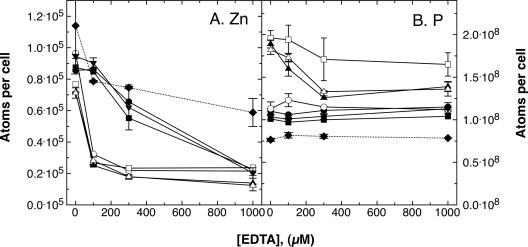
The effect of EDTA on the metal content of C. metallidurans was further analyzed using ICP-MS (Fig. 5). With increasing EDTA concentrations, the zinc contents of C. metallidurans strain CH34 wild type and the plasmid-free strain AE104 decreased gradually. E. coli W3110, which was used for comparison and possesses a ZnuABC and a ZupT system, contained a much higher zinc content at high EDTA concentrations than C. metallidurans. In contrast, the zinc content of the ΔzupT strain decreased sharply at low EDTA concentrations (Fig. 5A). The Ni and Co contents in all four strains decreased sharply at low EDTA concentrations, while those of the monovalent cations Na, K, Mn, Ca, and Fe did not change (data not shown). Thus, C. metallidurans CH34 and AE104 are able to obtain zinc under conditions of low availability, and ZupT is required for this feature.
Fig. 5.
Zinc and phosphate contents of C. metallidurans and E. coli cells. The contents of zinc and phosphate atoms per cell were determined by ICP-MS in C. metallidurans CH34 wild type (•), megaplasmid-free AE104 (▪), and the AE104 ΔzupT (○) and ΔzupT ΔpitA (□) mutant strains, as well as E. coli strain W3110 (♦). The cells were cultivated for 20 h in TMM with shaking at 30°C. Three independent determinations were made. The error bars indicate standard deviations.
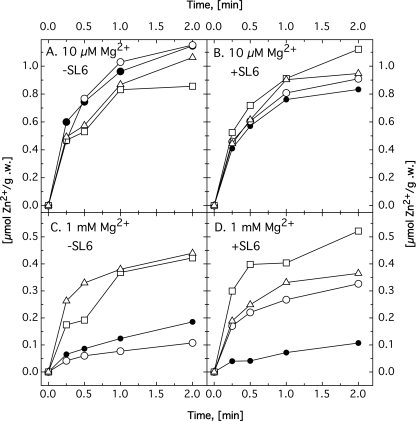
Uptake of zinc was more closely examined using 65Zn2+ (Fig. 6 and Table 3). Comparing the 65Zn2+ uptake of strain AE104 parent cells under various conditions reproduced a 10-fold-increased zinc uptake under conditions of magnesium starvation (41) and about 50% increase of zinc uptake when the cells were cultivated in the absence of trace element solution SL6. Deletion of zupT did not impair the ability of the cells to take up 65Zn2+ under conditions of sufficient zinc in the environment. Under magnesium starvation, uptake of zinc by the ΔzupT mutant cells was similar to that of the control cells (Fig. 6A and B). Probably, other systems were upregulated under these conditions, imported zinc efficiently, and led to a concomitant increase in the cellular cobalt content (Table 2).
Fig. 6.
Zinc uptake by C. metallidurans mutant strains. C. metallidurans strain AE104 (•) and its ΔzupT (○), ΔpitA (□), and ΔzupT ΔpitA (▵) derivatives were cultivated in TMM with decreased magnesium content, 10 μM (A and B) or 1 mM (C and D) Mg2+, either without (A and C) or with (B and D) the trace element solution SL6 in the medium. The cells were harvested by centrifugation, suspended in Tris buffer, and used in uptake experiments with 65Zn2+. Mean values of at least three experiments are shown. Error bars were omitted to avoid cluttering.
Table 3.
Uptake of 65Zn2+ by various C. metallidurans mutant strains
| Strain or mutant | Uptake of 65Zn2+a |
|||
|---|---|---|---|---|
| 10 μM Mg2+ |
1 mM Mg2+ |
|||
| Without SL6 | With SL6 | Without SL6 | With SL6 | |
| AE104 | 1.15 ± 0.60 | 0.83 ± 0.07 | 0.19 ± 003 | 0.11 ± 0.01 |
| ΔzupT | 1.15 ± 0.18 | 0.91 ± 0.13 | 0.11 ± 0.04 | 0.33 ± 0.26 |
| ΔpitA | 0.86 ± 0.03 | 1.12 ± 0.02 | 0.42 ± 0.13 | 0.52 ± 0.25 |
| corA1::kan | 1.10 ± 0.28 | 1.11 ± 0.18 | 0.22 ± 0.07 | 0.20 ± 0.06 |
| ΔcorA2 | 1.13 ± 0.03 | 1.00 ± 0.02 | 0.20 ± 0.06 | 0.22 ± 0.03 |
| ΔcorA3 | 1.45 ± 0.24 | 0.90 ± 0.10 | 0.18 ± 0.04 | 0.24 ± 0.07 |
| ΔzupT ΔpitA | 1.06 ± 0.34 | 0.95 ± 0.09 | 0.44 ± 0.34 | 0.37 ± 0.14 |
As for Fig. 6, C. metallidurans strain AE104 and its mutant derivatives were cultivated in TMM with decreased magnesium content (10 μM) (Fig. 6A and B) or 1 mM Mg2+ (Fig. 6C and D), either without (Fig. 6A and C) or with (Fig. 6B and D) the trace element solution SL6 in the medium. The cells were harvested by centrifugation, suspended in Tris buffer, and used in uptake experiments with 65Zn2+. The values at a t of 2 min are given in μmol 65Zn2+/g dry mass. At least 3 reproductions.
With sufficient magnesium and in cells cultivated without trace element solution SL6, zinc import of the ΔzupT mutants was 2-fold decreased compared to the parent strain, AE104, but 3-fold increased when the cells were cultivated in the presence of SL6 (Fig. 6C and D). Zinc import by ΔzupT cells without SL6 was very similar to that of AE104 parent cells with SL6, indicating that ZupT may have been responsible for 50% of the zinc import in the parent cells cultivated in the presence of high magnesium concentrations but without SL6, in agreement with the observed upregulation of zupT under conditions of zinc starvation (Fig. 2). The 3-fold increase in zinc import in the ΔzupT mutant at 1 mM magnesium plus SL6 might be explained by upregulation of other metal importers in the ΔzupT strain under these conditions.
Regulation of expression of pitA-lacZ, corA1-lacZ, corA2-lacZ, and corA3-lacZ by the external zinc concentration did not change in the ΔzupT strain compared to the parent control (Fig. 1). However, the pattern of the phosphate-dependent regulation of pitA-lacZ was different in the ΔzupT and the parent backgrounds (Fig. 3B): in both strains, a maximum expression level of 600 U/mg was reached at 5 mM phosphate. This level decreased 2-fold in the parent background at higher phosphate concentrations but remained at a high level in the ΔzupT mutant. Thus, PitA may have been able to substitute for ZupT with respect to zinc uptake at high phosphate concentrations and in the presence of SL6.
Effect of the pitA deletion on metal homeostasis.
Deletion of pitA did not change zupT-lacZ expression (Fig. 2B) or resistance to metal cations, to EDTA (Fig. 4; see Table S4 in the supplemental material), or to metal-EDTA complexes (see Table S5 in the supplemental material). The ΔpitA deletion strain accumulated more 65Zn2+ than the parental strain, AE104, when cultivated in sufficient magnesium (with or without SL6) but contained 25% less zinc when the cells were starved for magnesium and trace elements (Fig. 6 and Table 3). Thus, import of cation-phosphate complexes by PitA might be an important metal source; however, other zinc transfer systems were able to substitute for a missing PitA system. Regulation of expression of the three corA-lacZ fusions by zinc did not change in the ΔpitA mutant strain (Fig. 1).
Deletion of zupT and pitA.
The metal resistance of the ΔzupT ΔpitA double mutant was similar to that of the single mutants or the AE104 parent with the exception of decreased cobalt resistance (see Table S4 in the supplemental material). The resistance to EDTA of the double mutant was similar to that of the ΔzupT single mutant, whereas that of the ΔpitA single mutant was on the parental level. Further deletion of pitA did not change the EDTA sensitivity of the ΔzupT mutant. Addition of zinc (0.5 mol zinc per mol EDTA) completely abolished the EDTA sensitivity of the double mutant, similar to that of the ΔzupT single mutant. While cobalt (0.5 mol/mol EDTA) could also counteract the EDTA sensitivity of the ΔzupT single mutant, this was not observed for the double mutant (see Table S5 in the supplemental material). Cells of the double mutant showed 3-fold-increased magnesium and 2-fold-increased phosphate contents (Table 2 and Fig. 5B) compared to the parent strain, AE104, plus an increased cobalt content similar to that of ΔzupT cells. Deletion of pitA from the ΔzupT mutant obviously led to magnesium and phosphate starvation, perhaps leading to upregulation of additional metal and phosphate importers. Thus, PitA seemed to be an important magnesium-phosphate (in general, metal cation-phosphate) uptake system.
The zinc content, however, in cells of the ΔpitA ΔzupT double mutant was similar to that of the AE104 parent (Table 2). Similar to the ΔzupT single mutant, double-mutant cells were unable to obtain zinc from EDTA complexes (Fig. 5A). Uptake of zinc was on the same level as for the ΔpitA single-mutant strain (Fig. 6 and Table 3). Thus, if zinc was not sequestered, e.g., by EDTA, the double mutant still contained enough importers for zinc and the other metal cations. Since the zinc-dependent downregulation of the three corA-lacZ reporter fusions was not changed in the double mutant compared to the parental background (Fig. 1), this indicates the presence of unknown uptake systems.
When strain AE104 and the ΔzupT single- and the ΔzupT ΔpitA double-mutant strains were cultivated under conditions of multiple-metal stress, strain AE104 maintained metal ion homeostasis at 10 μM (each) of the five metal cations but started to lose control and accumulate sodium at 30 μM. Growth stopped at 100 μM (see Table S6 in the supplemental material). The deletion strains did not even survive at 30 μM. The double mutant was less able to cope with multiple-cation stress at 10 μM each metal than the ΔzupT single mutant. This indicated that both systems were required for full metal ion resistance already in the plasmid-free strain AE104.
Single corA gene deletions.
In agreement with the proposed function as the main secondary magnesium uptake system, magnesium resistance in the ΔcorA1 mutant strain increased from a MIC of 55 mM (strain AE104) to 70 mM (data not shown). Since corA1 expression was upregulated when cells were cultivated in the presence of 10 μM Mg2+ (Fig. 5A), CorA1 could serve as a zinc importer under these conditions. However, uptake of 65Zn2+ in ΔcorA1, ΔcorA2, or ΔcorA3 single-deletion strains was not compromised (Table 3).
Deletion of any corA gene did not change the metal resistance of strain AE104 or expression of zupT-lacZ significantly (Table 3 and Fig. 2). Deletion of corA1, but not of the other two corA genes, led to decreased EDTA resistance, similar to deletion of zupT (Fig. 4; see Table S4 in the supplemental material). However, and in contrast to ΔzupT, any metal tested increased EDTA resistance when added at 0.5 mol/mol EDTA, with magnesium being less efficient than transition metal cations (see Table S5 in the supplemental material). Probably, each of the metals mobilizes sufficient zinc or other metals from the EDTA complexes to be taken up by ZupT or PitA. Thus, none of the three CorA systems was required as long as the other two CorA proteins, PitA and ZupT, were present in the cell.
DISCUSSION
Living with many metals.
C. metallidurans strain CH34 is a facultative chemolithoautotrophic bacterium that has evolved to become superbly adapted to environments containing high concentrations of transition metals (19, 57). When the metal contents of C. metallidurans strains CH34 and AE104 and of E. coli strain W3110 were compared, their metal contents increased linearly with the outside concentration, and there was not much difference between the sensitive strains AE104 and W3110 and the resistant strain CH34, indicating that metal resistance did not influence the cellular metal content. This suggested that binding of the metals to the surfaces of the bacteria contributed to the measured values. Nevertheless, zinc binding to cells of strain CH34 requires living cells and is therefore an active process (25). Since strain AE104 did not display similarly increased zinc-binding properties (see Table S2 in the supplemental material), increased zinc binding should also be a plasmid-mediated activity. The many periplasmic proteins that are encoded by parts of the czc operon may be involved in this process (40, 63).
The upper concentration limits of metal tolerance also became visible when cells were simultaneously treated with five toxic metals. Strain CH34 tolerated up to 100 μM each of the five metals and started to show growth inhibition at this concentration, strain AE104 did not grow at 100 μM but did at 30 μM, and the ΔzupT and ΔzupT ΔpitA strains did not grow at 30 μM. Both deletion strains continued to grow at 10 μM, but with enhanced accumulation of other metals in the case of the double mutant. Therefore, the functions of at least these two uptake systems were required for parallel handling of five toxic metals.
Below this upper metal tolerance limit and under most conditions tested, a high concentration of one metal in the growth medium did not influence the cellular content of another. This might be accomplished by “metal homeostasis shunts,” combinations of uptake and efflux systems assigned to each metal and regulated in their expression and activities by the metal (see Fig. S1, inset, in the supplemental material). Indeed, the previously identified efflux systems can be assigned to a single central metal cation as a substrate, so they could play their part should such a shunt exist (see Fig. S1 in the supplemental material). The three PIB2-type ATPases (TC 3.A.3) ZntA, CadA, and PbrA remove Zn2+, Cd2+, and Pb2+, respectively, from the cytoplasm (4, 7, 24, 49, 51, 56). The CDF proteins (TC 2.A.3) DmeF and FieF export Co2+/Ni2+ or Fe2+, respectively (12, 32, 51), into the periplasm. From there, the RND-driven (TC 2.A.6) transenvelope efflux systems CzcCBA and CnrCBA transport Co2+/Zn2+/Cd2+ or Co2+/Ni2+, respectively, from the periplasm to the outside (37). The PIB4-type ATPase CzcP, the CDF protein CzcD, and the DMT protein CnrT (TC 2.A.7) enhance export of Zn2+, Co2+/Zn2+/Cd2+, or Ni2+, respectively, from the cytoplasm to the periplasm to feed additional substrate cations into the RND-driven efflux systems (2, 3, 51). Up to four PIB1-type ATPases, two RND-driven efflux systems, and a variety of other proteins maintain copper homeostasis (31) and may also be involved in silver and gold transformation (22, 47).
This arrangement assigns a basal export system plus a system for high metal concentrations to each individual metal cation: for Zn2+, these are ZntA (basal) plus CzcP/CzcD/CzcCBA (high); for Cd2+, CadA (basal) plus CzcD/CzcCBA (high); for Ni2+, DmeF (basal) plus CnrT/CnrCBA (high); and for Co2+, DmeF (basal) plus CzcD/CzcCBA/CnrCBA (high). Moreover, backup systems are ready to compensate for failure of the main systems. ZntA, CadA, and PbrA may substitute for each other, and CzcP and many RND-driven system may have broad substrate specificities when on a higher expression level (cobalt for CzcP and zinc for CnrCBA) or are only expressed to phenotypic relevance after a mutation (40, 51, 57).
There are no metal homeostasis shunts.
Now, do the uptake systems line up with the efflux systems to form a shunt? They do in E. coli, and ABC uptake systems are needed for this. Examples are ZnuACB for zinc (44), NikABC for nickel (33), and ModABCD for molybdate (46, 52). ZnuABC, ZntA, and their regulators Zur and ZntR form a shunt as described above, controlling the cytoplasmic zinc content in the bacterium (43). NickABC, RncA, NikR, and RcnR could be a second shunt (17, 21), albeit linked to iron and cobalt homeostasis.
However, there are no known ABC uptake systems for divalent metal cations in C. metallidurans, and none of the five systems ZupT, PitA, and CorA1, CorA2, and, CorA3 could be assigned to a single metal so that a specific homeostasis shunt of an uptake and efflux system is formed. The only regulatory signals in control of the expression levels of the five systems were zinc starvation for zupT, magnesium starvation for corA1, and the zinc concentration in general for all five systems. Other metals, such as Co2+, Ni2+, and Cd2+, did not regulate the expression of any of the studied genes under the conditions tested. Nevertheless, these metals are bound to the C. metallidurans cell (Table 2) and were imported (41). High zinc concentrations downregulated expression of all five genes studied but did not decrease their expression level to zero. Thus, for most metal cations, no “shunt” seems to exist.
C. metallidurans seems not to use much manganese.
This leaves the “worry later” hypothesis: highly redundant uptake systems with low ion selectivity take up what they can get and leave it to the more specific efflux systems to remove anything in surplus.
However, not anything is being taken up. C. metallidurans does not contain a known manganese efflux system—no SitABC-type and no MntH manganese/cadmium importer of the NRAMP protein family—as E. coli does. Consequently, the manganese content of E. coli cells grown in TMM was 12-fold higher than that of strain CH34. When challenged with 30 μM cadmium, E. coli contained many more atoms of cadmium per cell than CH34 or AE104 and started to lose control of its metal homeostasis system (see Table S2 in the supplemental material), probably indicating cytoplasmic accumulation of the metal (14, 15). The main Mn-containing enzyme of E. coli is the superoxide dismutase SodA (38). The main superoxide dismutase in CH34, however, is an iron-containing SodB-type protein (48), while the only Mn-containing SOD-like protein, ChrC, has very low activity and is part of the chromate detoxification system (20). This indicated that CH34 has no MntH and with it the highly active housekeeping protein SodA, presumably to decrease the uptake of the nonspecific MntH substrate cadmium. Thus, absence of MntH in strain CH34 may be a prerequisite for its cadmium resistance, in addition to the possession of CadA, CzcD, and CzcCBA.
Functions of the five uptake systems.
ZupT is likely the main uptake system for Zn2+ under conditions of zinc starvation, such as in the presence of EDTA. On the other hand, ZupT is not the only system able to import zinc. When C. metallidurans strain AE104 was cultivated under conditions of magnesium starvation, deletion of zupT or pitA or double deletion of both genes did not change zinc uptake by the cells (Fig. 6). Obviously, magnesium starvation led to upregulation of a transport system that imported zinc. This “magnesium uptake system” was assigned to metal uptake in C. metallidurans previously (41). Since deletion of the genes for any of the transport systems tested did not hamper zinc import in cells grown under magnesium starvation (Table 3 and Fig. 6), PitA, ZntA, or the three CorA proteins are not this “magnesium uptake system.”
As judged by the activity of the reporter gene fusion, pitA had the highest expression level among the five studied systems. Its deletion led to increased ability for zinc import into cells of strain AE104. While deletion of zupT doubled the amount of cell-bound cobalt, the additional deletion of pitA also increased that of phosphate and magnesium (Table 2). Increase in the phosphate content (Fig. 5) can be explained by upregulation of the phosphate-specific system PstABC, which is encoded on chromosome 1 by the putative operon Rmet_2179 to Rmet_2185 and includes the genes for an ABC transport system, the periplasmic phosphate-binding protein PhoS, and the regulatory factors PhoU, PhoB, and PhoR. The higher magnesium content may be the result of upregulation of CorA1 or the unknown “magnesium uptake system.” Thus, upregulation of PitA by high phosphate concentrations (Fig. 3B) indicated that PitA is a metal-phosphate uptake system. This has been observed before for E. coli (18). The affinity of metals for phosphate from the solubility product constants (http://www.ktf-split.hr/periodni/en/abc/kpt.html) indicates that most metal cations may be available as parts of phosphate complexes in TMM. Thus, PitA may be an important metal importer in bacteria. CorA1 seemed to be a central magnesium uptake system, and CorA2/CorA3 seem to be backup systems.
Taking the data together, in C. metallidurans, adaptation to metal-rich environments seems to have led to a genetic outfit with uptake systems with rather broad substrate specificity that import metal phosphate complexes (PitA), magnesium and other metals (CorA1), or zinc and other metals (ZupT), leaving it to the cell to “worry later” what to do with a nonoptimal cytoplasmic metal ion bouquet. Here, efflux systems control and regulate the content and bouquet composition of this metal pool (see Fig. S2 in the supplemental material). In addition, C. metallidurans contains a variety of RND-driven efflux pumps that may adjust the periplasmic concentrations of the transition metals, thus dealing with the substrates of the uptake systems. This action may lead to substrate competition between these uptake systems and RND-driven pumps for metals. In this model, the intrinsic function of ZupT would be to deal with this situation.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this work was provided to D.H.N. and G.G. by the Deutsche Forschungsgemeinschaft (Ni262/10).
We thank Grit Schleuder and Karola Otto for skillful technical assistance. D.H.N. thanks G.G. and the staff at the School of Biological Sciences, University of Nebraska, Lincoln, NE, for accommodating him at his sabbatical after his service time as dean was over. The stay in Lincoln was sponsored by the Institute for Ecoremediation, and the sabbatical work contributed to the production of this article.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Albert H., Dale E. C., Lee E., Ow D. W. 1995. Site-specific integration of DNA into wild-type and mutant lox sites placed in the plant genome. Plant J. 7:649–659 [DOI] [PubMed] [Google Scholar]
- 2. Anton A., Große C., Reißman J., Pribyl T., Nies D. H. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 181:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anton A., et al. 2004. Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans and Escherichia coli. J. Bacteriol. 186:7499–7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argüello J. M., Eren E., Gonzalez-Guerrero M. 2007. The structure and function of heavy metal transport P-1B-ATPases. Biometals 20:233–248 [DOI] [PubMed] [Google Scholar]
- 5. Bachmann B. J. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36:525–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beard S. J., et al. 2000. Evidence for the transport of zinc(II) ions via the Pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol. Lett. 184:231–235 [DOI] [PubMed] [Google Scholar]
- 7. Borremans B., Hobman J. L., Provoost A., Brown N. L., Van der Lelie D. 2001. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 183:5651–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diels L., Mergeay M. 1990. DNA probe-mediated detection of resistant bacteria from soils highy polluted by heavy metals. Appl. Environ. Microbiol. 56:1485–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eitinger T., Suhr J., Moore L., Smith J. A. C. 2005. Secondary transporters for nickel and cobalt ions: theme and variations. Biometals 18:399–405 [DOI] [PubMed] [Google Scholar]
- 10. Goldberg M., Pribyl T., Juhnke S., Nies D. H. 1999. Energetics and topology of CzcA, a cation/proton antiporter of the RND protein family. J. Biol. Chem. 274:26065–26070 [DOI] [PubMed] [Google Scholar]
- 11. Goris J., et al. 2001. Classification of metal-resistant bacteria from industrial biotopes as Ralstonia campinensis sp. nov., Ralstonia metallidurans sp. nov. and Ralstonia basilensis Steinle et al. emend. Int. J. Syst. Evol. Microbiol. 51:1773–1782 [DOI] [PubMed] [Google Scholar]
- 12. Grass G., et al. 2005. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 183:9–18 [DOI] [PubMed] [Google Scholar]
- 13. Haber F., Weiss J. 1932. Über die Katalyse des Hydroperoxydes. Naturwissenschaften 20:948–950 [Google Scholar]
- 14. Helbig K., Bleuel C., Krauss G. J., Nies D. H. 2008. Glutathione and transition metal homeostasis in Escherichia coli. J. Bacteriol. 190:5431–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helbig K., Grosse C., Nies D. H. 2008. Cadmium toxicity in glutathione mutants of Escherichia coli. J. Bacteriol. 190:5439–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irving H., Williams R. J. P. 1948. Order of stability of metal complexes. Nature 162:746–747 [Google Scholar]
- 17. Iwig J. S., Rowe J. L., Chivers P. T. 2006. Nickel homeostasis in Escherichia coli—the rcnR-rcnA efflux pathway and its linkage to NikR function. Mol. Microbiol. 62:252–262 [DOI] [PubMed] [Google Scholar]
- 18. Jackson R. J., et al. 2008. Expression of the PitA phosphate/metal transporter of Escherichia coli is responsive to zinc and inorganic phosphate levels. FEMS Microbiol. Lett. 289:219–224 [DOI] [PubMed] [Google Scholar]
- 19. Janssen P. J., et al. 2010. The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS One 5:e10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juhnke S., Peitzsch N., Hübener N., Große C., Nies D. H. 2002. New genes involved in chromate resistance in Ralstonia metallidurans strain CH34. Arch. Microbiol. 179:15–25 [DOI] [PubMed] [Google Scholar]
- 21. Koch D., Nies D. H., Grass G. 2007. The RcnRA (YohLM) system of Escherichia coli: a connection between nickel, cobalt and iron homeostasis. Biometals 20:759–771 [DOI] [PubMed] [Google Scholar]
- 22. Ledrich M. L., Stemmler S., Laval-Gilly P., Foucaud L., Falla J. 2005. Precipitation of silver-thiosulfate complex and immobilization of silver by Cupriavidus metallidurans CH34. Biometals 18:643–650 [DOI] [PubMed] [Google Scholar]
- 23. Lee S. M., et al. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215:273–278 [DOI] [PubMed] [Google Scholar]
- 24. Legatzki A., Anton A., Grass G., Rensing C., Nies D. H. 2003. Interplay of the Czc-system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 185:4354–4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legatzki A., et al. 2003. First step towards a quantitative model describing Czc-mediated heavy metal resistance in Ralstonia metallidurans. Biodegradation 14:153–168 [DOI] [PubMed] [Google Scholar]
- 26. Lenz O., Schwartz E., Dernedde J., Eitinger T., Friedrich B. 1994. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J. Bacteriol. 176:4385–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marx C. J., Lidstrom M. E. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062–1067 [DOI] [PubMed] [Google Scholar]
- 28. Mergeay M., et al. 2003. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 27:385–410 [DOI] [PubMed] [Google Scholar]
- 29. Mergeay M., et al. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monchy S., et al. 2007. Plasmids pMOL28 and pMOL30 of Cupriavidus metallidurans are specialized in the maximal viable response to heavy metals. J. Bacteriol. 189:7417–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monchy S., et al. 2006. Transcriptomics and proteomic analysis of the pMOL30-encoded copper resistance in Cupriavidus metallidurans strain CH34. Microbiology 152:1765–1776 [DOI] [PubMed] [Google Scholar]
- 32. Munkelt D., Grass G., Nies D. H. 2004. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 186:8036–8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro C., Wu L. F., Mandrand-Berthelot M. A. 1993. The nik operon of Escherichia coli encodes a periplasmic binding-protein-dependent transport-system for nickel. Mol. Microbiol. 9:1181–1191 [DOI] [PubMed] [Google Scholar]
- 34. Nies D., Mergeay M., Friedrich B., Schlegel H. G. 1987. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J. Bacteriol. 169:4865–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nies D. H. 2007. Bacterial transition metal homeostasis, p. 118–142 In Nies D. H., Silver S. (ed.), Molecular microbiology of heavy metals, vol. 6 Springer-Verlag, Berlin, Germany [Google Scholar]
- 36. Nies D. H. 1992. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 174:8102–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nies D. H. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313–339 [DOI] [PubMed] [Google Scholar]
- 38. Nies D. H., Grass G. 1 October 2009, posting date Chapter 5. 4. 4. 3, Transition metal homeostasis. In Böck A., et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 39. Nies D. H., Nies A., Chu L., Silver S. 1989. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc. Natl. Acad. Sci. U. S. A. 86:7351–7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nies D. H., Rehbein G., Hoffmann T., Baumann C., Grosse C. 2006. Paralogs of genes encoding metal resistance proteins in Cupriavidus metallidurans strain CH34. J. Mol. Microbiol Biotechnol. 11:82–93 [DOI] [PubMed] [Google Scholar]
- 41. Nies D. H., Silver S. 1989. Metal ion uptake by a plasmid-free metal-sensitive Alcaligenes eutrophus strain. J. Bacteriol. 171:4073–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nies D. H., Silver S. 1989. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J. Bacteriol. 171:896–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Outten C. E., O'Halloran T. V. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492 [DOI] [PubMed] [Google Scholar]
- 44. Patzer S. I., Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199–1210 [DOI] [PubMed] [Google Scholar]
- 45. Pfennig N. 1974. Rhodopseudomonas globiformis, Sp-N, a new species of Rhodospirillaceae. Arch. Microbiol. 100:197–206 [Google Scholar]
- 46. Rech. S., Wolin C., Gunsalus R. P. 1996. Properties of the periplasmic ModA molybdate-binding protein of Escherichia coli. J. Biol. Chem. 271:2557–2562 [DOI] [PubMed] [Google Scholar]
- 47. Reith F., et al. 2009. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. U. S. A. 106:17757–17762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roux M., Coves J. 2002. The iron-containing superoxide dismutase of Ralstonia metallidurans CH34. FEMS Microbiol. Lett. 210:129–133 [DOI] [PubMed] [Google Scholar]
- 49. Saier M. H. J., Tran C. V., Barabote R. D. 2006. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 34:D181–D186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning, a laboratory manual, 2nd. ed. Cold Spring Harbor Laboratory., Cold Spring Harbor, NY [Google Scholar]
- 51. Scherer J., Nies D. H. 2009. CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol. Microbiol. 73:601–621 [DOI] [PubMed] [Google Scholar]
- 52. Schwarz G., Hagedoorn P.-L., Fischer K. 2007. Molybdate and tungstate: uptake, homeostasis, cofactors, and enzymes. In Nies D. H., Silver S. (ed.), Molecular microbiology of heavy metals, vol. 6 Springer, Berlin, Germany [Google Scholar]
- 53. Simon R., Priefer U., Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 54. Snavely M. D., Florer J. B., Miller C. G., Maguire M. E. 1989. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by CorA, MgtA, and MgtB systems. J. Bacteriol. 171:4761–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suzuki N., Nonaka H., Tsuge Y., Inui M., Yukawa H. 2005. New multiple-deletion method for the Corynebacterium glutamicum genome, using a mutant lox sequence. Appl. Environ. Microbiol. 71:8472–8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taghavi S., et al. 2009. Lead(II) resistance in Cupriavidus metallidurans CH34: interplay between plasmid and chromosomally-located functions. Antonie Van Leeuwenhoek 96:171–182 [DOI] [PubMed] [Google Scholar]
- 57. von Rozycki T., Nies D. H. 2009. Cupriavidus metallidurans: evolution of a metal-resistant bacterium. Antonie Van Leeuwenhoek 96:115–139 [DOI] [PubMed] [Google Scholar]
- 58. von Rozycki T., Nies D. H., Saier M. H. J. 2005. Genomic analyses of transport proteins in Ralstonia metallidurans. Comp. Funct. Genomics 6:17–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Waldron K. J., Rutherford J. C., Ford D., Robinson N. J. 2009. Metalloproteins and metal sensing. Nature 460:823–830 [DOI] [PubMed] [Google Scholar]
- 60. Wan Q., et al. 2011. X-ray crystallography and isothermal titration calorimetry studies of the Salmonella zinc transporter ZntB. Structure 19:700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weast R. C. 1984. CRC handbook of chemistry and physics, 64th ed. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 62. Worlock A. J., Smith R. L. 2002. ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:4369– 4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zoropogui A., Gambarelli S., Coves J. 2008. CzcE from Cupriavidus metallidurans CH34 is a copper-binding protein. Biochem. Biophys. Res. Commun. 365:735–739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.