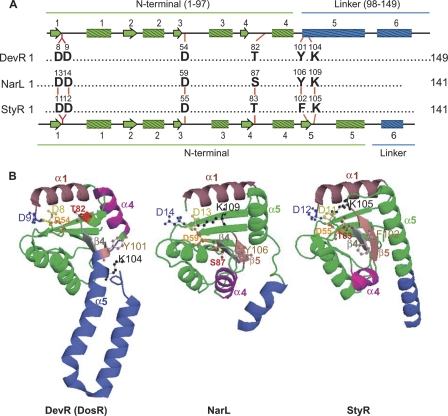
Fig. 1.
Activation pocket in DevR (DosR), NarL, and StyR. (A) Structure-based alignment of the conserved residues in the activation pocket of NarL subfamily members. A schematic representation of the secondary structure elements of N-terminal (green) and linker (blue) domains of DevR is shown on top of the alignment, and that for NarL and StyR is shown below (45). Residue numbers are indicated on the top of each residue. (B) Comparison of structures of unphosphorylated DevR (PDB3C3W), NarL (PDB1A04), and StyR (PDB1ZN2). The corresponding α-helices, β-sheets, and the conserved residues of the activation pocket in these proteins have been color matched and labeled. StyR and NarL display the classical (βα)5 fold of receiver domains. The structures were generated from the PDB files using program PyMol.