Fig. 1.
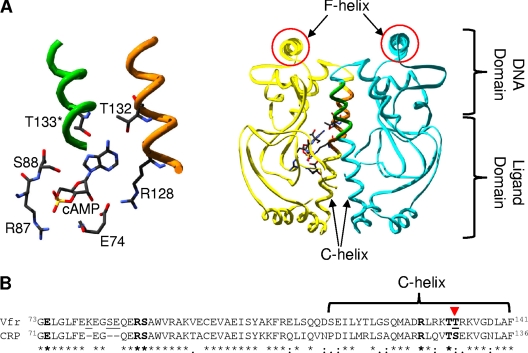
(A) cAMP-bound structure of Vfr (PDB ID 2OZ6) with cAMP-contacting residues highlighted. The right side shows the overall structure, while the cAMP pocket is enlarged and rotated slightly in the left panel. The cAMP-contacting residues shown are all from one subunit except for Thr133 (T133). The protein functions as a dimer (one part in yellow and the other in blue). The two F-helices (the DNA-contacting regions) are circled in red. The figure was visualized using Swiss-PdbViewer, version 4.01, and POV-Ray, version 3.6.2. (B) Alignment of ligand-binding domains of Vfr (NP_249343.1) and CRP (NP_417816.1), which cover the known primary cAMP-binding pocket residues. The known cAMP-contacting residues in both proteins are shown in boldface, and the two proteins differ only in one residue that is indicated by the inverted triangle. The T-Coffee program was used to generate the alignment. In the alignment, an asterisk (*) indicates the same amino acid in the two proteins, a colon (:) indicates a conservative substitution, and a period (.) indicates a semiconservative substitution.