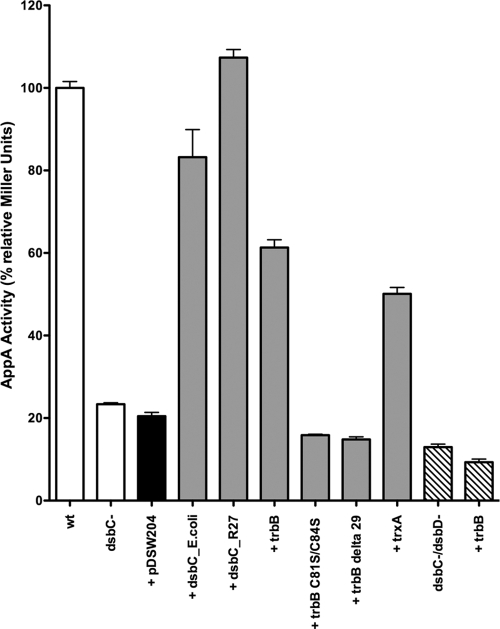
Fig. 3.
Examination of disulfide isomerase activity by AppA pNPP hydrolysis. AppA requires a disulfide isomerase to fold properly; therefore, pNPP hydrolysis is an indirect measure of disulfide isomerase activity. AppA activity is shown for a ΔdsbCE.coli mutant (black bar) complemented with an empty plasmid (pDSW204) or plasmids encoding disulfide isomerase DsbCE.coli, putative disulfide isomerases DsbCR27 and TrbB, TrbB N-terminal truncation mutant TrbBΔ29, TrbB active site mutant TrbB C81S/C84S, and reductase TrxA (gray bars). AppA activity in a ΔdsbCE.coli ΔdsbD double mutant strain, with or without TrbB, was also assessed (striped bars). DHB4 (wild-type [wt]) activity was set to 100%.