Figure 6.
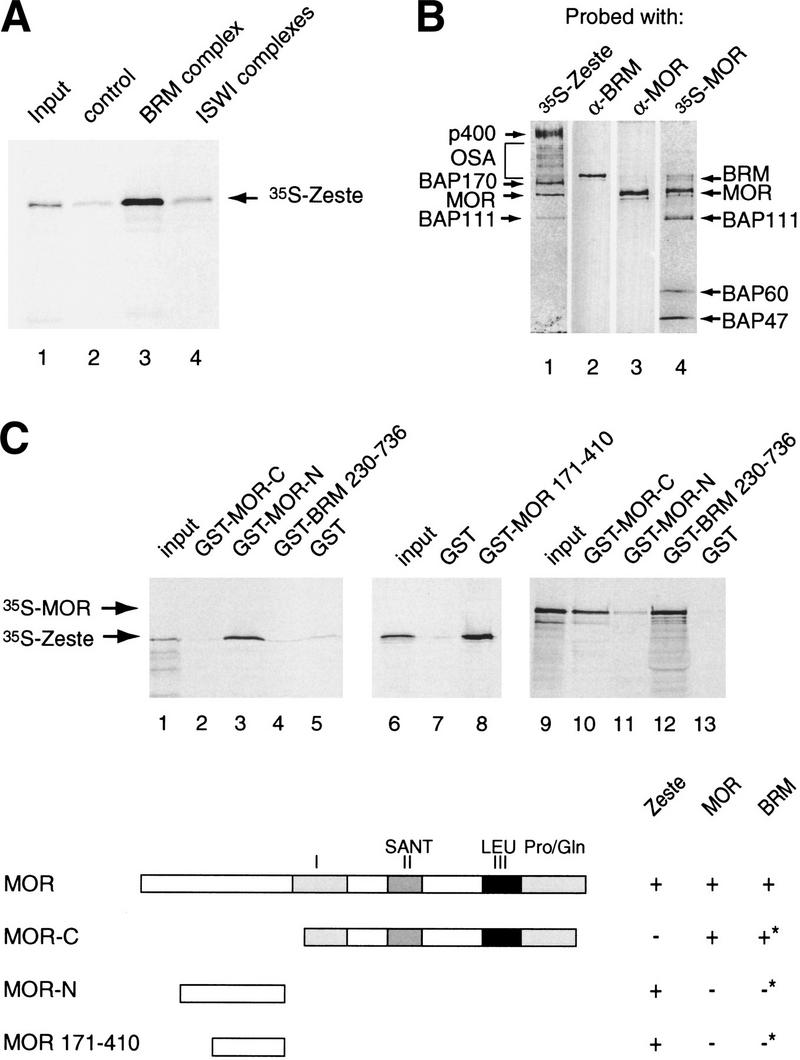
Zeste directly binds selected BAPs within the BRM complex. (A) Zeste interacts with the BRM complex. Protein-A Sepharose resin (control, lane 2), anti-BRM affinity beads loaded with BRM complex (lane 3), or anti-ISWI affinity beads loaded ISWI complexes (lane 4) were incubated with 35S-labeled reticulocyte expressed Zeste. Protein complexes were washed, resolved by SDS-PAGE, and bound Zeste was detected by autoradiography. Lane 1 represents 5% of the input material used in the binding reactions. (B) Far-Western blotting analysis reveals that Zeste targets specific BAPs. The purified BRM complex (monoS #42) was resolved by SDS-PAGE and transferred to nitrocellulose. The nitrocellulose membrane was treated with 6 m guanidine-HCl, renatured, washed, and incubated with 35S-labeled reticulocyte expressed Zeste (lane 1) or MOR (lane 4). After extensive washing the filter was exposed to film. Filter 1 and 4 were reprobed with antibodies directed against BRM (lane 2) or MOR (lane 3), respectively. The positions of the BAPs bound by Zeste or MOR are indicated on the left (Zeste) or right (MOR) of the panels. (C) Mapping of the Zeste binding domain of MOR by GST pull-down assays. GST-MOR carboxyl terminus (residues 454–1174; lanes 2,10), GST-MOR amino terminus (residues 106–410; lanes 3,11), GST-MOR (residues 171–410; lane 8), GST-BRM (residues 230–736; lanes 4,12), or GST alone (lanes 5,7,13) were immobilized on glutathione-Sepharose beads and incubated with either 35S-Zeste (lanes 2–8) or 35S-MOR (lanes 10–13). Protein complexes were washed, resolved by SDS-PAGE and bound proteins were detected by autoradiography. Lanes 1, 6, and 9 represent 5% of the input material used in the binding reactions. The domain structure of MOR and its interaction domains with itself, BRM (asterisk indicates data from Crosby et al. 1999) and Zeste are indicated.
