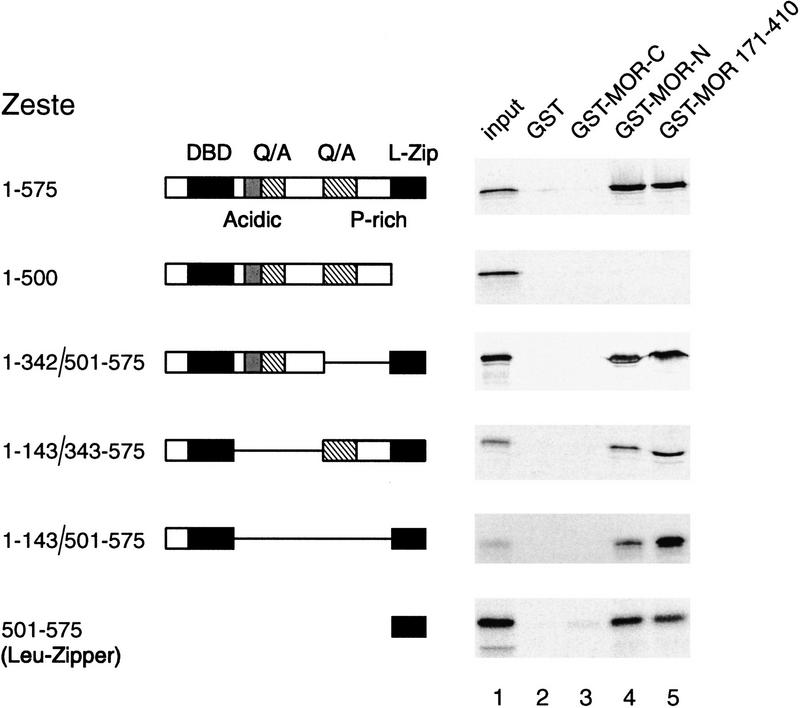
Figure 7.
The leucine zipper of Zeste binds MOR. Mapping of the MOR-binding domain of Zeste by GST pull-down assays. GST alone (lane 2), GST-MOR carboxyl terminus (lane 3), GST-MOR amino terminus (lane 4), or GST-MOR residues 171–410 (lane 5) were immobilized on glutathione-Sepharose beads and incubated with 35S-methionine-labeled Zeste or various Zeste deletion mutants. Protein complexes were washed, resolved by SDS-PAGE, and bound proteins were detected by autoradiography. Lane 1 represents 5% of the input material used in the binding reactions. The domain structure of Zeste and the amino acid residues present in the various deletion mutants are indicated (DBD) DNA-binding domain; (L-zip) leucine zipper; (Q/A) region rich in glutamines and alanines; (AD) acidic domain; (P-rich) proline rich domain.