Fig. 2.
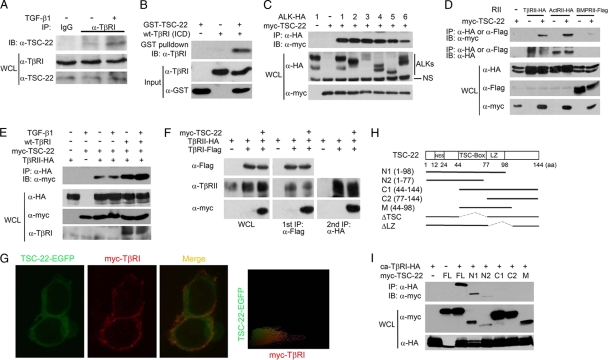
TSC-22 directly interacts with TβRI. (A) TGF-β enhances the TβRI-TSC-22 interaction at the endogenous protein level. HEK293 cells were treated with or without 100 pM TGF-β1 for 2 h and harvested for immunoprecipitation (IP) with control rabbit IgG or anti-TβRI antibody followed by anti-TSC-22 immunoblotting (IB). WCL, whole-cell lysate. (B) Direct interaction between TSC-22 and the intracellular domain (ICD) of TβRI. Purified recombinant GST-TSC-22 and TβRI-ICD (1 μg each) were subjected to GST pulldown assays. (C) TSC-22 associates with the type I receptors of the TGF-β family. After transfection with the indicated constructs (2 μg each) for 48 h, HEK293T cells were harvested for anti-hemagglutinin (anti-HA) immunoprecipitation (IP) followed by anti-Myc immunoblotting (IB). Protein expression was examined with whole-cell lysates (WCL) by immunoblotting. (D) TSC-22 associates with the type II receptors of the TGF-β family. (E) TGF-β and TβRI enhance the association between TSC-22 and TβRII. After transfection with indicated constructs for 48 h, HEK293T cells were treated with 100 pM TGF-β1 for 2 h before being harvested for coimmunoprecipitation. (F) TSC-22 forms a ternary complex with TβRI and TβRII. HEK293T cells transfected with indicated constructs were harvested for the first immunoprecipitation with anti-Flag antibody. The immunocomplex was then eluted by the use of Flag peptide and subjected to anti-HA immunoprecipitation followed by immunoblotting (right panel). Aliquots of whole-cell lysates and the first immunoprecipitants were analyzed by immunoblotting (left and middle panels). (G) TSC-22 substantially colocalizes with TβRI along the plasma membrane. HEK293T cells transfected with TSC-22-enhanced GFP (TSC-22-EGFP) and myc-TβRI were treated with 100 pM TGF-β1 for 2 h before being harvested for immunofluorescence analysis. TSC-22 localization was detected by direct EGFP immunofluorescence, and TβRI localization was determined indirectly using anti-myc antibody followed by a tetramethyl rhodamine isocyanate (TRITC)-conjugated second antibody and visualized under a fluorescence microscope. Colocalization was measured by IPP software; yellow coloring indicates the colocalization. (H) Schematic diagram of TSC-22 and its mutants. (I) The N terminus of TSC-22 mediates its interaction with ca-TβRI. The experiment was performed as described for panel A.