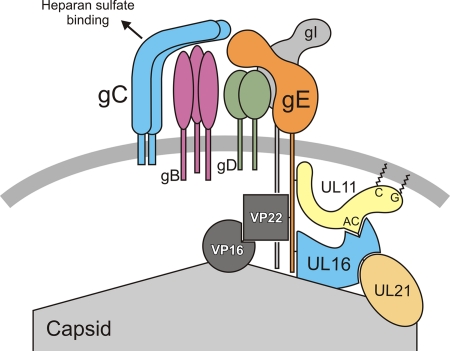
Fig. 10.
Model for the UL16-interaction network within virions. gE exists as a heterodimer with gI within a larger complex containing other viral glycoproteins. Binding of the virus to heparan sulfate via gC sends a signal that destabilizes the UL16-capsid interaction. That signal may be passed through gE, which has UL16 bound to its tail. UL16 also binds to an acidic cluster (AC) motif within UL11, which is peripherally associated with the membrane via an N-terminal glycine (G) modification with myristate and cysteine (C) modifications with palmitate. Moreover, UL11 may be associated with the tail of gE. For further details of this and other possible models, see the Discussion.