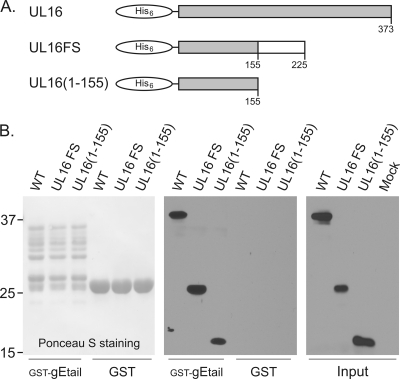
Fig. 7.
Discovery of a UL16-deletion mutant that retains the ability to bind gE. (A) Diagrams showing His6-UL16, the fortuitous frameshift mutant, and the construct containing only the first 155 residues. (B) Lysates from E. coli expressing wild-type (WT) or mutant His6-UL16 proteins were mixed with the indicated GST fusion proteins on glutathione beads and incubated to allow binding. The beads were washed to remove unbound proteins, and the samples were subjected to SDS-PAGE. The separated proteins were transferred to a nitrocellulose membrane, and the GST fusion proteins were visualized by Ponceau S staining. Bound and input His6-UL16 proteins were detected by immunoblotting with an antibody that recognizes the His6 tag.