Fig. 3.
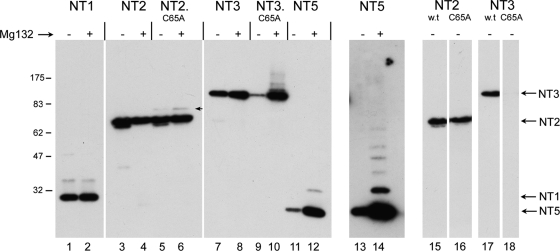
Dependence of N-terminal variants on USP activity for inhibition of ubiquitination and degradation. N-terminal variants of VP1-2 containing wt C65 or the C65A substitution as indicated (Fig. 1) were transfected into Cos cells and treated without or with addition of MG132 (added 4 h prior to harvesting). Mutation in the active site of the USP domain in NT2 resulted in a novel low-abundance species (arrowed lane 6) but had very little overall effect on NT2 levels (cf. lanes 3 and 5). Proteosome inhibition had little effect on total levels of either NT2 or NT2.C65A (lanes 3 to 6). In contrast, mutation in the USP active site resulted in a profound reduction in NT3 levels (cf. lanes 7 and 9 to shorter-exposure lanes 17 and 18). Moreover, proteosome inhibition restored levels of NT3.C65A and induced a series of poorly resolved higher-molecular-weight species (cf. lanes 9 and 10). NT5 was also poorly expressed with proteosome inhibition inducing a significant increase in levels and higher-molecular-weight species (cf. lanes 11 and 12) and longer exposure (lanes 13 and 14).