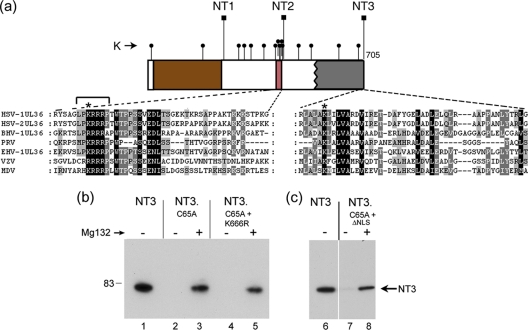
Fig. 6.
Single conserved lysine residues within the VP1-2 N terminus are not sufficient to mediate degradation of catalytically inactive variants. (a) Schematic of the N-terminal constructs NT1, NT2, and NT3, whose C-terminal endpoints are indicated (square lollipops). Lysine residues are indicated by small round lollipops. Sequence alignments of the N terminus of VP1-2 reveal a highly conserved lysine residue within the boundary between NT2 and NT3, indicated by an asterisk above the expanded section of sequence. Numerous lysine residues are present between the boundaries of NT1 and NT2, which also includes a demonstrated functional NLS (2). Within the NLS is located a conserved lysine, as indicated by an asterisk above the expanded sequence. (b) Substitution of these individual lysines does not result in significant stabilization of NT3.C65A. NT3, NT3.C65A, variants of NT3.C65A containing K666R, and a 7-residue deletion at the NLS (indicated by bracket above sequence alignment) were analyzed in the absence or presence of MG132 (added 8 h before harvesting). The double mutants behaved like NT3.C65A, being virtually undetectable in the absence of proteosome inhibition and stabilized by proteosome inhibition to approximately similar levels.