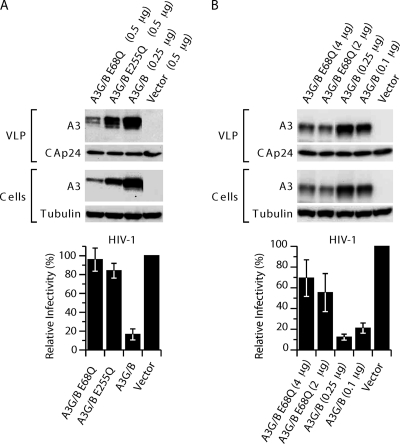
Fig. 4.
A3G/B inhibition of HIV-1 infection depends on catalytically active sites. (A) Single-cycle replication assays were performed with VLPs produced in 293T cells cotransfected with the A3G/B chimera and catalytically active site mutants, pCMVHIV-1, pUCHR-GFPLuc, and pCMV-VSV-G. HeLaP4 cells were infected with the resultant VLPs, and productive infection was measured by the luciferase activity. Values are presented as percent infectivity relative to virus produced in the absence of A3G/B and mutants. The data are the means from three independent experiments, and error bars represent the standard deviations. (B) Expression and virus packaging of A3 proteins and chimeras with catalytically active site mutations. The presence of A3 proteins in virus and cell extracts was analyzed by immunoblotting with anti-HA antibody probing. Tubulin and HIV-1 CA levels were detected to verify equal cell and virion extract loading.