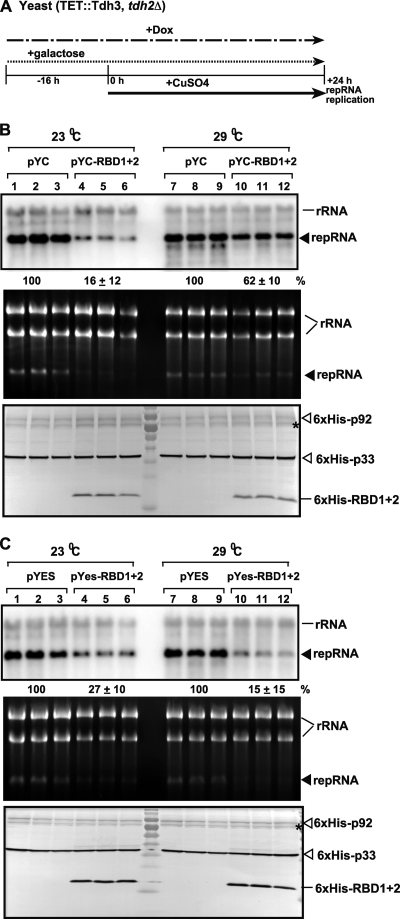
Fig. 5.
Inhibitory effect of GAPDH RBD1+2 dominant negative mutant overexpression on TBSV repRNA accumulation in yeast. (A) For these experiments, TET::TDH3 tdh2Δ yeast was used, which contains a doxycycline regulatable promoter replacing the native TDH3 promoter. Therefore, the addition of doxycycline to the culture media can downregulate GAPDH (Tdh3p) expression at the mRNA level. To launch TBSV repRNA replication, we expressed 6×His-p33 and 6×His-p92 from the copper-inducible CUP1 promoter and DI-72(+) repRNA from the galactose-inducible GAL1 promoter. First, the GAPDH level was reduced by adding doxycycline- and galactose-containing media to induce the expression of RBD1+2 at 16 h prior to expressing the viral components. Then, the yeast cells were cultured for 24 h at either 23 or 29°C on 2% galactose SC minimal medium containing doxycycline plus 50 μM CuSO4. TBSV repRNA accumulation was tested at the two different temperatures since 23°C is the most favorable for TBSV repRNA replication, while the expression of GAPDH is highest at 29°C. (B) For the top panel, Northern blot analysis was used to detect DI-72(+) repRNA accumulation in a yeast strain overexpressing RBD1+2 dominant negative mutant from the low-copy-number pYC plasmid as shown. For the middle panel, an ethidium-bromide stained gel of total RNA extracts of the samples was used for the Northern blotting described above. For the bottom panel, a Western blot analysis of the accumulation level of 6×His-tagged p33, 6×His-tagged p92, and 6×His-tagged RBD1+2 using anti-6×His antibody was performed. The asterisk (*) indicates an SDS-resistant p33 dimer. (C) The same experiments as in panel B, except that RBD1+2 was expressed from the high-copy-number pYES plasmid, as shown.