Abstract
The glycans on HIV-1 gp120 play an important role in shielding neutralization-sensitive epitopes from antibody recognition. They also serve as targets for lectins that bind mannose-rich glycans. In this study, we investigated the interaction of the lectin griffithsin (GRFT) with HIV-1 gp120 and its effects on exposure of the CD4-binding site (CD4bs). We found that GRFT enhanced the binding of HIV-1 to plates coated with anti-CD4bs antibodies b12 and b6 or the CD4 receptor mimetic CD4-IgG2. The average enhancement of b12 or b6 binding was higher for subtype B viruses than for subtype C, while for CD4-IgG2, it was similar for both subtypes, although lower than observed with antibodies. This GRFT-mediated enhancement of HIV-1 binding to b12 was reflected in synergistic neutralization for 2 of the 4 viruses tested. The glycan at position 386, which shields the CD4bs, was involved in both GRFT-mediated enhancement of binding and neutralization synergism between GRFT and b12. Although GRFT enhanced CD4bs exposure, it simultaneously inhibited ligand binding to the coreceptor binding site, suggesting that GRFT-dependent enhancement and neutralization utilize independent mechanisms. This study shows for the first time that GRFT interaction with gp120 exposes the CD4bs through binding the glycan at position 386, which may have implications for how to access this conserved site.
INTRODUCTION
HIV-1 gp120 is heavily glycosylated, and N-linked glycans account for ∼50% of its molecular mass (30, 32). Three types of glycans are found on gp120, namely, high-mannose glycans composed of 7 to 9 terminal mannose residues, complex glycans containing terminal sialic acid residues, and hybrid glycans, which are a mixture of both (9, 20, 29, 62). There are approximately 11 glycosylation sites on monomeric gp120 that are occupied by either mannose-rich or hybrid glycans, while the remaining sites bear complex glycans (32). However, recently Doores et al. reported that 98% of glycans on native HIV-1 envelope (Env) are barely processed beyond Man5GlcNAc2, i.e., glycans containing five mannose residues (16). Glycosylation patterns between HIV-1 subtype B and C envelopes have also been reported to differ in number and frequency (60). In addition to their role in promoting the proper folding of gp120 and mediating its interaction with cellular receptors, glycans protect HIV-1 from antibody neutralization by masking sensitive epitopes on the envelope (19, 34, 35, 37–39, 54, 55).
The CD4-binding site (CD4bs) on gp120 is highly conserved among HIV-1 subtypes and is a target for antibodies (29, 58). Among HIV-1 antibodies that target the CD4bs is the broadly neutralizing monoclonal antibody b12. The epitope of this antibody is located primarily in the neutralizing face of gp120, and 82% of its binding site is in the outer domain of the viral envelope (61). However, the high-mannose glycan at position 386 located inside the CD4bs shields this site from antibodies, as its removal has been shown to increase HIV-1 sensitivity to b12 (24, 48, 52, 66). In addition, the CD4bs is a target of nonneutralizing antibodies, such as b6. However, unlike b12, which binds both monomeric and trimeric gp120, b6 binds only to the monomeric form of the glycoprotein (48).
High-mannose glycans on gp120 are also targets of glycan-specific agents, such as lectins. Several lectins have been identified in recent years that potently block the infectivity of viruses, such as HIV and influenza virus (6, 41, 46). One of the most potent of these is griffithsin (GRFT). GRFT is a 121-amino-acid and ∼13-kDa-molecular-mass lectin that was originally isolated from the red alga Griffithsia sp. (41). GRFT exists exclusively as a dimer and has a domain-swapped structure in which two β-strands of one monomer combine with 10 β-strands of the other monomer to form a β prism of three four-stranded sheets (63, 64). Each GRFT monomer contains three binding sites that have high affinity for mannose residues. Both native and recombinant GRFT display potent antiviral activities against primary HIV-1 isolates by binding to high-mannose glycans on the viral envelope spike (41, 47). We previously showed that the 234 and 295 glycosylation sites play an important role in GRFT neutralization of HIV-1 (1).
Since GRFT binds high-mannose oligosaccharides, including the one at position 386 that conceals the b12 epitope, we wished to explore whether this lectin affected exposure of the CD4bs. We examined binding using both a virus capture assay and neutralization. We found that GRFT enhanced HIV-1 binding of b12 and the nonneutralizing CD4bs monoclonal antibody (MAb) b6, as well as CD4-IgG2, which was used here as a surrogate for the CD4 receptor molecule. Importantly, GRFT and b12 synergized to render some HIV-1 isolates more sensitive to neutralization. The glycan at position 386 on gp120 was found to play a role in both enhancement and synergy, suggesting that GRFT could be used to increase exposure of the CD4bs of HIV-1.
MATERIALS AND METHODS
Viruses and reagents.
HIV-1 subtype B envelope clones QH0692.42 and PVO.4, amplified from acutely infected individuals (33), were obtained from the NIH Reference and Reagent Program. The cloned subtype C envelopes COT9.6 and COT6.15 were derived from chronic pediatric infections (21), while Du151.2 and CAP239.G3J were amplified from acutely infected patients (23, 56). Infectious primary viruses were isolated from subtype B (DS12)- and subtype C (Du151 and CM9)-infected adults (12, 13), while RP1 was isolated from a chronic pediatric patient (21). The HIV-2 envelope clone 7312A was provided by George Shaw, and the pSG3Δenv plasmid was obtained from Beatrice Hahn. The MAbs IgG1b12 (b12) and IgG1b6 (b6) were kindly provided by Dennis Burton. MAbs 4E10, F240, and 17b were obtained from the NIH Reference and Reagent Program. The subtype C-specific anti-V3 MAb 3468L was isolated from an HIV-positive patient (22). The anti-CCR5 inhibitor PRO140, the CD4 receptor surrogate CD4-IgG2, and soluble CD4 (sCD4) were generously provided by Progenics Pharmaceuticals, Inc. (Tarrytown, NY). Recombinant GRFT and CV-N were purified from Escherichia coli at the National Cancer Institute, Frederick, MD (6, 41).
Generation of env-pseudotyped viruses.
HIV-1 pseudoviruses were generated by cotransfection of the gp41-gp120 (Env) and pSG3Δenv plasmids (55) into 293T cells using the Fugene transfection reagent (Roche Applied Science, Indianapolis, IN). The 50% tissue culture infective dose (TCID50) of each virus stock was determined by infecting TZM-bl cells with serial 5-fold dilutions of the supernatant in quadruplicate in the presence of DEAE dextran (37.5 μg/ml) (Sigma-Aldrich, St. Louis, MO). The Bright Glo Reagent (Promega, Madison, WI) was used to measure infection after 48 h of culture, according to the manufacturer's instructions. Luminescence was measured in a Wallac 1420 Victor Multilabel Counter (Perkin-Elmer, Norwalk, CT). The TCID50 values were calculated as described elsewhere (27).
Site-directed mutagenesis.
N-linked glycosylation site signal sequences were introduced into HIV-1 gp120 by QuikChange Site Directed Mutagenesis (Stratagene, LaJolla, CA). The presence of the mutation was confirmed by sequencing using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA) and resolved on the ABI 3100 automated genetic analyzer.
Single-cycle neutralization assay (TZM-bl assay).
Pseudovirus neutralization assays were performed as described elsewhere (40). Briefly, a 3-fold dilution series of GRFT in 100 μl of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) (growth medium) was prepared in a 96-well plate in duplicate. Two hundred TCID50 of pseudovirus in 50 μl of growth medium was added, and the mixture was incubated for 1 h at 37°C. Then, 100 μl of TZM-bl cells at a concentration of 1 × 105 cells/ml containing 37.5 μg/ml of DEAE dextran was added to each well and cultured at 37°C for 48 h. Infection was evaluated by measuring the activity of the firefly luciferase. Titers were calculated as the inhibitory concentration that causes a 50% reduction (IC50) in relative light units (RLU) compared to the virus control (wells with no inhibitor) after the subtraction of the background (wells without both the virus and the inhibitor).
To measure synergism, HIV-1 was incubated with a 3-fold dilution series of GRFT and b12 in 100 μl of DMEM-10% FBS. The lectin and the MAb were diluted alone and in combination. This was followed by the addition of the virus to TZM-bl cells. The IC50 and IC80 of GRFT and b12 were determined in the wells containing each compound alone and in the wells containing a mixture of both compounds. Synergism between GRFT and HIV-positive plasma or serum was measured as for b12, except that the 50% infective dose (ID50) and ID80 were calculated. Since PRO140 is a CCR5 inhibitor, TZM-bl cells were first incubated with a dilution series of the compound prior to the addition of the virus with or without GRFT (in a dilution series) to allow for PRO140 binding to the coreceptor. The IC50 and IC80 of the lectin and PRO140 when used alone and in combination were calculated. Synergism was determined by calculating the combination index (CI) using both the IC50 and the IC80 (11, 59). A CI of 0.3 to 0.7 was deemed indicative of synergism, 0.7 to 0.85 of moderate synergism, 0.85 to 0.9 of slight synergism, and 0.9 to 1.1 of an additive effect, as previously defined (11, 67).
GRFT inhibition of HIV-1 infection of U87-CCR5 and U87-CXCR4.
U87-CCR5 or U87-CXCR4 at a concentration of 2 × 104 cells/well were cultured for 24 h in a flat-bottom plate. This was followed by the addition of HIV-1 primary isolates that were preincubated for 1 h with a 3-fold dilution series of GRFT in 150 μl of DMEM with 10% FBS. The following day, the cells were washed three times and cultured for 10 days. The percent inhibition of infection was determined by comparing the p24 concentration of GRFT-containing wells to the control wells. The IC50 of GRFT inhibition of HIV-1 was calculated by plotting the lectin concentration against the percent inhibition in a linear regression using GraphPad Prism 4.0.
HIV-1 virion capture assay.
A high-binding 96-well plate (Corning Inc., Corning, NY) was coated overnight with 100 μl/well of a 10-μg/ml solution of b12, b6, F240, 4E10, 3468L, or CD4-IgG2 in NaHCO3 (pH 8.5). The coated plate was washed three times with phosphate-buffered saline (PBS) and blocked with 3% bovine serum albumin (BSA) in PBS at 37°C for 2 h. HIV-1 was incubated for an hour with GRFT, CV-N, CD4-IgG2, or the MAbs, and the virus was then added to the plate and left at 37°C for 2 h. The plate was washed three times with PBS, captured virus was lysed with 150 μl of 0.5% Triton X-100, and the p24 concentration was measured by enzyme-linked immunosorbent assay (ELISA). Control wells contained HIV-1 captured in the absence of the lectins, MAbs, or CD4-IgG2.
The assay was modified to assess competition between GRFT and 17b for binding to the CD4-induced epitope (CD4i) using the HIV-2 virus 7312A in the presence of sCD4 (15). After 1 h incubation with different concentrations of the lectin, the virus was incubated with 25 μg/ml of sCD4 for another 1 h before addition to a 17b-coated plate for 2 h. After the plate was washed three times with PBS, the amount of captured p24 was quantified as explained above. In another version of the same experiment, the virus was incubated first with 25 μg/ml of sCD4 and then with different concentrations of the lectin before addition to the 17b-coated plate for 2 h. For GRFT competition with 17b, using a plate coated with CD4-IgG2, the virus was first incubated with the CD4-IgG2-coated plate for 1 h. This was followed by the sequential addition of 25 μg/ml of sCD4 (to expose the CD4i on Env spikes that did not bind to the surface-bound CD4-IgG2); then by 30, 6, or 1.2 μg/ml of 17b; and thereafter by 30 μg/ml of GRFT, each for 1 h. The plate was washed with PBS three times between incubations. Rabbit anti-GRFT polyclonal antibodies and a horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody were used to measure the amount of GRFT bound to the virus using an optical density of 450 nm.
RESULTS
HIV-1 binding to b12 and b6 MAbs was enhanced in the presence of GRFT.
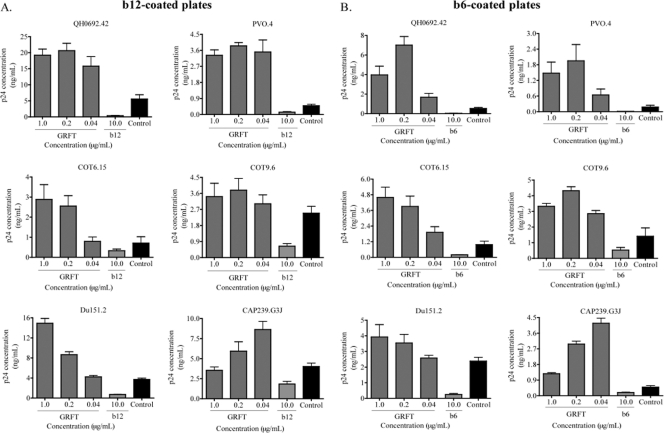
We previously showed that GRFT competes with the glycan-specific MAb 2G12 for binding to HIV-1 (1). During this investigation, we observed that GRFT enhanced the binding of another anti-HIV MAb, b12, which targets the CD4bs and is broadly neutralizing (4, 5). In order to further explore this observation, we made use of a virus capture assay (VCA) that is highly reproducible and commonly used (8, 10, 45, 50). Most importantly, VCA offers a simple way of evaluating HIV-1 interaction with both neutralizing and nonneutralizing antibodies, as both capture viruses (10), although some gp41 antibodies have been reported to be captured only weakly (31). However, this assay has the disadvantage of being unable to provide information about the neutralization capacity of the antibody studied, so in this study, we performed both VCA and neutralization in parallel. We used two subtype B (QH0692.42 and PVO.4) and four subtype C (COT6.15, COT9.6, Du151.2, and CAP239.G3J) pseudoviruses, which were preincubated with GRFT and then added to b12-coated plates. Captured viruses were lysed, and the amount of p24 antigen was measured relative to wells with no lectin. As shown in Fig. 1A, preincubation with GRFT significantly enhanced virus capture by the b12 MAb above control b12-coated wells with no GRFT. The greatest effect was observed with the 2 subtype B viruses: for PVO.4, it was 8-fold above the control, and for QH0692.42, it reached 4-fold. The effect was slightly less pronounced for the four subtype C viruses: for Du151.2 and COT6.15, binding increased by 4-fold, while for CAP239.G3J, binding increased by 2-fold, and for COT9.6, the increase was 1.5-fold above the control.
Fig. 1.
HIV-1 capture by MAbs b12 and b6 was enhanced in the presence of GRFT. HIV-1 env-pseudotyped viruses from subtype B (QH0692.42 and PVO.4) and subtype C (COT6.15, COT9.6, Du151.2, and CAP239.G3J) were incubated with three different concentrations of GRFT before being added to plates coated with either b12 (A) or b6 (B). The amount of captured virus was assessed by p24 ELISA. Each MAb was also competed against itself as a positive control. The controls (black bars) show the amounts of virus captured on b12- or b6-coated plates in the absence of GRFT. The bars represent the means and standard deviations (SD) of three different experiments.
In order to determine if this GRFT enhancement of binding was specific to the b12 MAb, we tested another CD4bs MAb called b6, which is nonneutralizing despite its epitope overlapping with that of b12 (48, 51). GRFT enhanced the binding of all six viruses to b6, and the effect was even more striking than with b12 (Fig. 1B). Again, the subtype B viruses showed higher levels of binding than the subtype C viruses. The subtype B virus QH0692.42 exceeded the control by 13-fold, while among subtype C viruses, CAP239.G3J was the highest, reaching 6.2 times the control. For both b12 and b6, most viruses reached maximum enhancement between 0.2 and 1 μg/ml of GRFT, suggesting saturation of CD4bs exposure with little additional effect at higher concentrations. In most cases, a reduction in binding was observed for both b12 and b6 at 0.04 μg/ml, indicating a dose-response effect. CAP239.G3J reached maximal enhancement at the lowest GRFT concentration tested, perhaps because of its unusual sensitivity to GRFT compared to other subtype C viruses (see Table 5). Both b12 and b6 competed against themselves, as expected (Fig. 1A and B).
Table 5.
Synergy between GRFT and PRO140 for neutralization of HIV-1
| HIV-1 envelope pseudovirus | Single |
Combined |
CIa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GRFT |
PRO140 |
GRFT |
PRO140 |
IC50 | IC80 | |||||
| IC50 (nM) | IC80 (nM) | IC50 | IC80 | IC50 | IC80 | IC50 | IC80 | |||
| PVO.4 | 0.1 | 0.1 | 9.4 | 33.5 | 0.1 | 0.1 | 2.3 | 5.1 | 1.2 | 1.2 |
| QH0692.42 | 0.1 | 0.3 | 79.2 | 263.9 | 0.1 | 0.2 | 6.3 | 19.3 | 1.1 | 0.7 |
| COT6.15 | 0.8 | 2.6 | 21.2 | 128.6 | 0.3 | 0.6 | 13.1 | 46.4 | 1.0 | 0.6 |
| Du151.2 | 1.5 | 2.3 | 16.0 | 41.5 | 0.3 | 0.6 | 13.4 | 30.5 | 1.0 | 1.0 |
| CAP239.G3J | 0.2 | 0.9 | 36.2 | 165.8 | 0.2 | 0.4 | 6.5 | 22.1 | 1.2 | 0.6 |
| COT9.6 | 2.7 | 6.3 | 31.0 | 147.2 | 0.5 | 1.6 | 23.8 | 53.0 | 1.0 | 0.6 |
A CI of 0.3 to 0.7 indicates synergism, 0.7 to 0.85 indicates moderate synergism, 0.85 to 0.9 indicates slight synergism, and 0.9 to 1.1 indicates an additive effect. Cases where synergism occurred are in boldface.
Since the CD4bs overlaps both the b12 and b6 epitopes, we also studied whether GRFT increased CD4 receptor binding to HIV-1 gp120. To achieve this, we carried out a capture assay using the chimeric molecule CD4-IgG2 and the viruses QH0692.42 and COT6.15, which showed the highest enhancement of b12 and b6 binding. As shown in Fig. 2, the presence of GRFT modestly enhanced capture with CD4-IgG2, and this enhancement reached ∼2 times the control for both viruses.
Fig. 2.
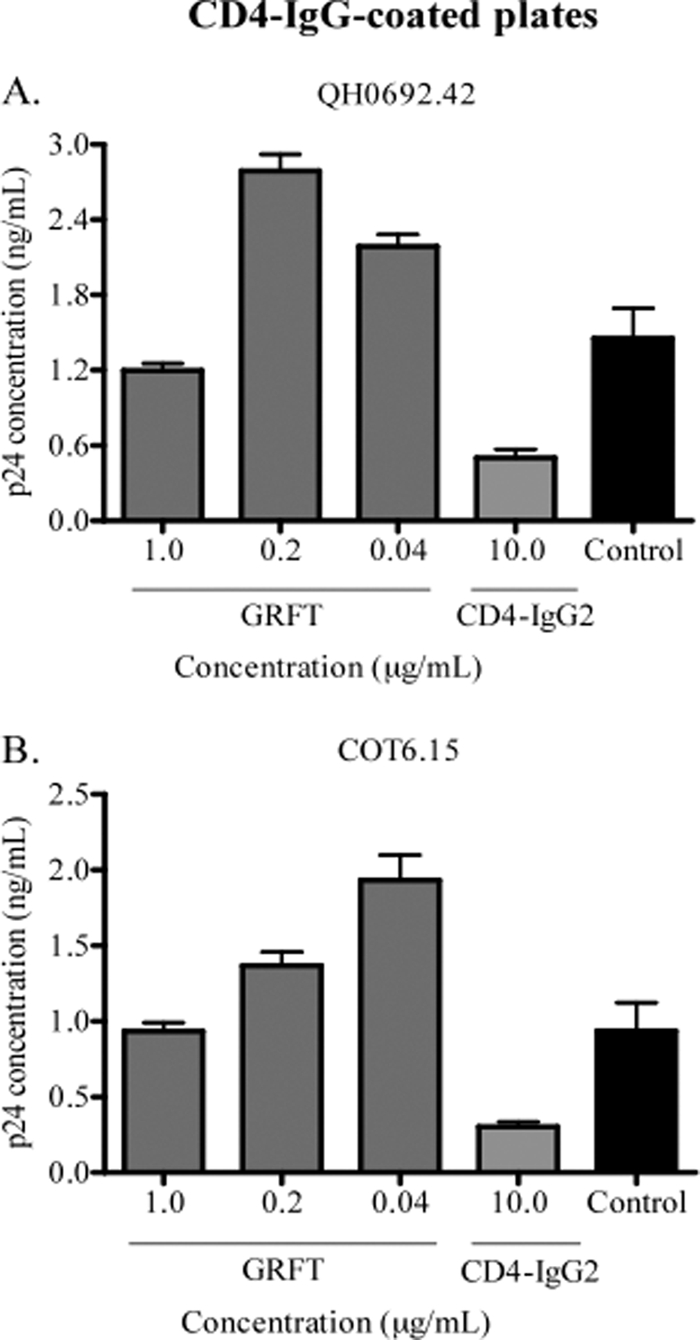
CD4-IgG2 capture of HIV-1 was enhanced by GRFT. HIV-1 subtype B pseudovirus QH0692.42 (A) and the subtype C pseudovirus COT6.15 (B) were incubated with different concentrations of GRFT and added to a plate coated with CD4-IgG2. The amount of captured virus was assessed by p24 ELISA relative to an untreated control with no GRFT (black bars). CD4-IgG2 was also competed against itself as a positive control. The bars represent the means and SD of three different experiments.
Enhancement of b12 and b6 binding was specific to GRFT and does not involve HIV-1 virion cross-linking.
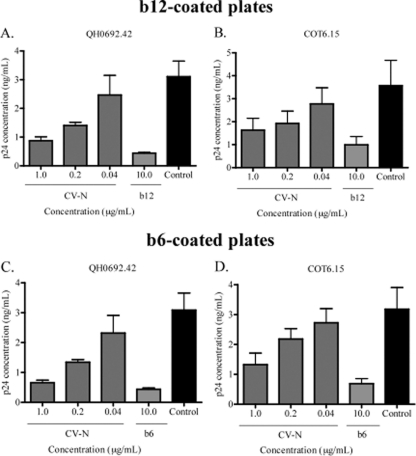
In order to explore the specificity of the observed enhanced exposure of the CD4bs by GRFT, we turned to CV-N, another mannose-specific lectin with strong anti-HIV neutralizing activity (64). QH0692.42 and COT6.15 were captured with either b12 or b6 in the presence of CV-N. Contrary to the observations with GRFT, CV-N blocked virus capture by both antibodies (Fig. 3A to D), showing that the ability of GRFT to enhance the binding of b12 and b6 to HIV-1 was specific to this lectin.
Fig. 3.
CV-N competed with b12 and b6 for binding to HIV-1. The subtype B pseudovirus QH0692.42 and the subtype C pseudovirus COT6.15 were incubated with different concentrations of CV-N and then added to plates coated with either b12 (A and B) or b6 (C and D). The amount of captured virus was assessed by p24 ELISA relative to an untreated control with no CV-N (black bars). MAbs were also competed against themselves as positive controls. The bars represent the means and SD of three different experiments.
Next, we determined whether the GRFT-mediated enhancement of b12 and b6 binding was the result of cross-linking of viral particles via the mannose residues. If so, similar enhancement should be observed if b12 or b6 were substituted for another antibody whose epitope does not overlap the CD4bs. We selected the MAbs F240 and 4E10, which bind gp41 at the immunodominant epitope and the membrane-proximal external region (MPER), respectively (7, 36), and 3468L, a MAb that binds the V3 loop (22), as the substitute antibodies. An ELISA plate was coated with them, and the pseudoviruses QH0692.42, PVO.4, and Du151.2, which had been preincubated with different concentrations of GRFT, were added. We observed that unlike b12 and b6, GRFT had no effect on virus binding to F240- and 4E10-coated plates and that p24 levels were equivalent to those of control wells without GRFT (Fig. 4A and B show data for 1 representative virus). There was also no enhanced binding of viruses to the anti-V3 MAb-coated plate, but unexpectedly, GRFT competed with 3468L for binding to these viruses (Fig. 4C). Nevertheless, these data indicated that the GRFT-mediated enhancement of binding to gp120 is specific to CD4bs antibodies and does not involve HIV-1 virion cross-linking by the lectin.
Fig. 4.
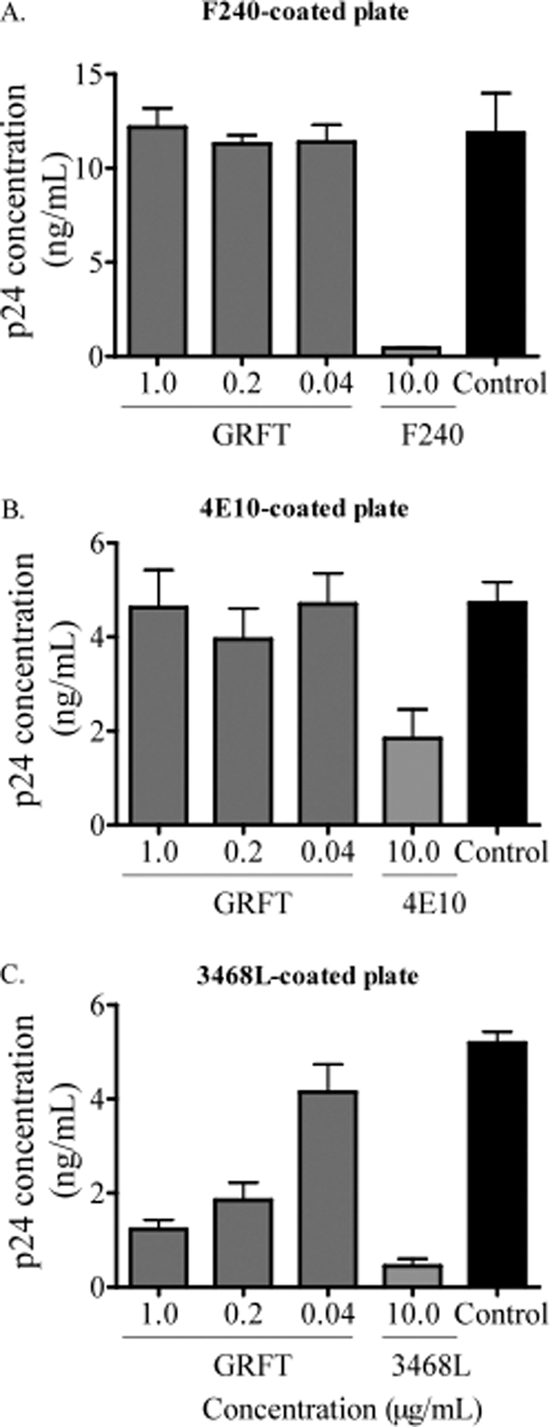
No enhancement of HIV-1 binding to F240-, 4E10-, and 3468L-coated plates by GRFT. The subtype B virus QH0692.42 was incubated with different concentrations of GRFT and then added to plates coated with the MAbs F240 (A), 4E10 (B), and 3468L (C). The amount of captured virus was assessed by p24 ELISA relative to an untreated control with no GRFT (black bars). Each antibody was also competed against itself as a positive control. The bars represent the means and SD of three different experiments.
The glycan at position 386 was involved in GRFT-mediated enhancement of HIV-1 binding to b12 and b6.
In order to determine if the number or position of mannose-rich glycans influenced the GRFT-mediated enhancement of b12 and b6 binding, we compared the glycosylation sites of all the viruses used in this study (Table 1). We observed that, except for COT9.6 and Du151.2, which lacked one and three glycans, respectively, all viruses lacked two glycosylation sites, suggesting that the number of glycans was not responsible for the variance in enhancement observed with these viruses. Three of the 4 subtype C viruses lacked the glycan at position 295, which is typical of viruses from this subtype (60). All six viruses, however, had intact glycosylation sites at positions 241, 262, 332, and 386.
Table 1.
Mannose-rich glycosylation pattern and GRFT-mediated enhancement of HIV-1 binding to b12 and b6
| HIV-1 envelope pseudovirus (subtype)a | Predicted N-linked mannose-rich glycosylation siteb |
Fold increase with GRFT at 0.2 μg/ml |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 230 | 234 | 241 | 262 | 289 | 295 | 332 | 339 | 386 | 392 | 448 | b12-coated plate | b6-coated plate | |
| PVO.4 (B) | x | x | 8.2 ± 2.1 | 11.1 ± 0.9 | |||||||||
| QH0692.42 (B) | x | x | 4.1 ± 1.5 | 13.5 ± 1.7 | |||||||||
| COT6.15 (C) | x | x | 4.5 ± 1.9 | 4.5 ± 1.8 | |||||||||
| Du151.2 (C) | x | x | x | 2.3 ± 0.04 | 1.5 ± 0.2 | ||||||||
| CAP239.G3J (C) | x | x | 1.5 ± 0.4 | 6.2 ± 1.2 | |||||||||
| COT9.6 (C) | x | 1.5 ± 0.06 | 3.9 ± 2.1 | ||||||||||
Viruses are ranked by subtype and according to their enhancement by GRFT.
Mannose-rich glycosylations were identified from the amino acid sequence of each envelope clone (32). Missing sites are marked by x.
We and others have previously shown that removal of the glycan at position 386 in HIV-1 gp120 increases the virus sensitivity to b12 neutralization (17, 24, 52). We therefore investigated whether this glycan might be involved in the GRFT-mediated enhancement of b12 and b6 binding to HIV-1. We deleted this site in PVO.4 and QH0692.42, and in both cases, the deletion resulted in a roughly 2-fold decrease in GRFT-mediated enhancement of binding to b12 and b6 (Table 2), suggesting that the 386 glycosylation site was involved in the GRFT-mediated effect.
Table 2.
Effects of glycan mutations on GRFT-mediated enhancement of binding to b12 and b6
| HIV-1 envelope pseudovirus | Predicted N-linked mannose-rich glycosylation sitesa |
Fold increase with GRFT at 0.2 μg/ml |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 230 | 234 | 241 | 262 | 289 | 295 | 332 | 339 | 386 | 392 | 448 | b12-coated plate | b6-coated plate | |
| PVO.4 | x | x | 8.2 ± 2.1 | 11.1 ± 0.9 | |||||||||
| PVO-N386Q | x | x | x | 4.8 ± 2.0 | 4.9 ± 1.9 | ||||||||
| QH0692.42 | x | x | 4.1 ± 1.5 | 13.5 ± 1.7 | |||||||||
| QH0692-N386Q | x | x | x | 2.8 ± 0.3 | 6.5 ± 2.7 | ||||||||
| COT6.15 | x | x | 4.5 ± 1.9 | 4.5 ± 1.8 | |||||||||
| COT6-V295N/S448N | 3.8 ± 0.02 | 8.7 ± 1.8 | |||||||||||
| COT6-V295N/S448N/N386Q | x | 1.7 ± 0.4 | 7.2 ± 2.8 | ||||||||||
| COT9.6 | x | 1.5 ± 0.06 | 3.9 ± 2.1 | ||||||||||
| COT9-V295N | 2.8 ± 0.3 | 7.7 ± 3.2 | |||||||||||
| COT9-V295N/N386Q | x | 1.3 ± 0.2 | 1.7 ± 0.2 | ||||||||||
Mannose-rich glycosylations were identified from the amino acid sequence of each envelope clone (32). Missing mannose-rich glycosylation sites are marked by x.
Since subtype C viruses showed lower levels of GRFT-mediated enhancement of b12 and b6 binding than subtype B viruses, we reconstituted all the mannose-rich glycans on COT6.15 and COT9.6 to determine if this increased the levels of binding. Addition of the 295N and 448N glycans to the COT6.15 envelope resulted in ∼2-fold-greater enhancement of binding to b6, while for COT9.6, addition of 295N increased both b6 and b12 binding compared to the corresponding wild-type viruses. Confirming what was seen for the 2 subtype B viruses, deletion of the 386 glycosylation site in these reconstituted subtype C viruses resulted in a similar decrease in the enhancement of b12 binding (Table 2).
GRFT synergized with b12 to inhibit HIV-1 infection.
To determine whether GRFT enhancement of b12 binding to HIV-1 translated into a synergistic interaction between the two compounds, we infected TZM-bl cells in the presence of GRFT and b12, individually and in combination. PVO.4 and CAP239.G3J were not neutralized by b12, and the presence of the MAb did not affect their sensitivity to GRFT, so synergy could not be measured. Viral infection was quantified after 48 h, and synergism between the antibody and the lectin at IC50 and IC80 was measured by calculating the CI (59). Both GRFT and b12 when tested individually neutralized all four pseudoviruses (Table 3). We observed synergism between b12 and GRFT for the neutralization of QH0692.42 and COT6.15, but not for COT9.6 or Du151.2. The CI values for QH0692.42 (at a GRFT/b12 molar ratio of 1.2 to 1) were 0.6 at IC50 and IC80, and for COT6.15 (at a GRFT/b12 molar ratio of 1 to 16.5), it was 0.7 at IC50. We were not able to calculate the CI value at IC80 for COT6.15, as the highest inhibition of the virus with b12 was 65% (data not shown). Lastly, we tested for synergism between GRFT and b6 for the neutralization of the six viruses mentioned above, and we observed no synergism (data not shown). In addition, the presence of b6 did not affect the sensitivity of these viruses to the lectin (data not shown).
Table 3.
Synergy between GRFT and b12 for neutralization of HIV-1
| HIV-1 envelope pseudovirus | Single |
Combined |
CIa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GRFT |
b12 |
GRFT |
b12 |
|||||||
| IC50b (μg/ml) | IC80c (μg/ml) | IC50 (μg/ml) | IC80 (μg/ml) | IC50 (μg/ml) | IC80 (μg/ml) | IC50 (μg/ml) | IC80 (μg/ml) | IC50 (μg/ml) | IC80 (μg/ml) | |
| QH0692.42 | 0.002 | 0.008 | 1.2 | 2.8 | 0.001 | 0.005 | 0.09 | 0.05 | 0.6 | 0.6 |
| QH0692-N386Q | 0.004 | 0.03 | 0.6 | 2.0 | 0.004 | 0.02 | 0.003 | 0.2 | 1.0 | 0.8 |
| COT6.15 | 0.06 | 0.2 | 33.6 | NDd | 0.03 | 0.1 | 5.6 | 18.6 | 0.7 | ND |
| COT6-V295N/S448N | 0.003 | 0.008 | 44.1 | ND | 0.002 | 0.006 | 0.02 | 0.9 | 0.7 | ND |
| COT6-V295N/S448N/N386Q | 0.001 | 0.004 | 1.1 | 3.8 | 0.002 | 0.3 | 0.02 | 11.0 | 2.0 | 1.0 |
| Du151.2 | 0.03 | 0.1 | 0.3 | 0.7 | 0.03 | 0.1 | 0.3 | 0.9 | 1.3 | 2.2 |
| COT9.6 | 0.1 | 0.4 | 1.7 | 9.0 | 0.06 | 0.3 | 2.2 | 11.0 | 1.9 | 2.0 |
A CI of 0.3 to 0.7 indicates synergism, 0.7 to 0.85 indicates moderate synergism, 0.85 to 0.9 indicates slight synergism, and 0.9 to 1.1 indicates an additive effect. Cases where synergism occurred are in boldface.
The IC50 is the concentration needed to reduce HIV-1 infection by 50%.
The IC80 is the concentration needed to reduce HIV-1 infection by 80%.
ND, not determined.
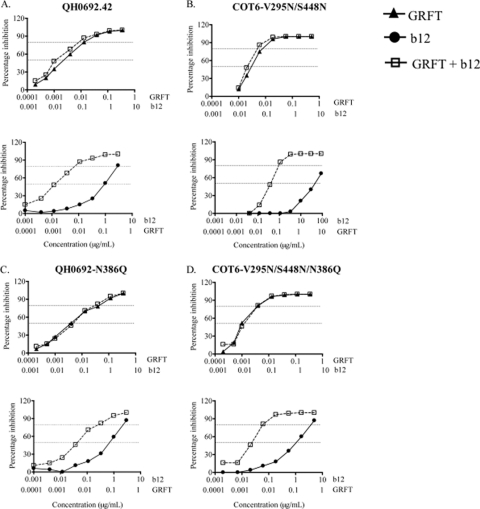
Since we showed that the glycan at position 386 was involved in the GRFT-mediated enhancement of binding, we investigated its effect on the observed synergy. For this experiment, we used QH0692.42 and the COT6.15 virus reconstituted with the 295N and 448N glycans, which showed the same level of synergy as the parental virus (Table 3). The N386Q mutant in this reconstituted virus was, however, considerably more sensitive to b12, as previously reported (Table 3) (24). For both QH0692-N386Q and COT6-V295N/S448N/N386Q, there was a loss of synergism between GRFT and b12 at IC50, implicating the 386 glycan in the synergistic interaction between GRFT and b12 for neutralization of the viruses tested.
To determine which compound benefited the most in this synergistic interaction, we analyzed the shift in the inhibition curves for GRFT and b12, when used alone or in combination. As shown in Fig. 5A and B, the combination of GRFT and b12 shifted the inhibition curves to the left relative to the curves for the single compounds. This suggested that QH0692.42 and COT6-V295N/S448N were more sensitive to each compound when used in combination. These observed shifts, while not statistically significant, were consistent. For b12, the IC50s were 13-fold lower in the presence of GRFT, while for GRFT, they were 2-fold lower, suggesting that b12 benefited the most from this combination. The deletion of the 386 glycan in both viruses resulted in a loss of synergy. This was shown by overlapping of the curves for GRFT when used either singly or in combination with b12 (Fig. 5C and D). In agreement with what we observed previously, the effect of the removal of the 386 glycan on GRFT neutralization curves was consistent but minor and not statistically significant. The curves for b12 were also affected; thus, for QH0692-N386Q, there was a 63% reduction in the shift between the single and combination curves for b12, while for COT6-V295N/S448N/N386Q, there was a 40% reduction despite the increased sensitivity of the virus to b12. These data confirm the involvement of the 386 glycan in GRFT and b12 synergistic interaction.
Fig. 5.
Effects of the 386 glycosylation site on the HIV-1 inhibition curves of GRFT and b12. (A and B) HIV-1 QH0692.42 (A) and COT6-V295N/S448N (B) were neutralized in TZM-bl cells with GRFT and b12, in combination and alone. The shift in the inhibition curves of the two compounds combined relative to each compound alone are shown. (C and D) The same experiment as in panels A and B but using QH0692-N386Q and COT6-V295N/S448N/N386Q, respectively. The dashed lines indicate 50 and 80% inhibition. The graphs are representative of three different experiments.
Synergy between GRFT and anti-CD4bs plasma antibodies.
Since plasma from some HIV-infected individuals contains antibodies that target the CD4bs (25), we investigated whether GRFT affects ID50 titers for the neutralization of HIV-1. We used HIV-positive plasma from BB10 that was shown to contain antibodies to the CD4bs and compared it to BB34, which contained anti-MPER antibodies (25). TZM-bl cells were infected with HIV-1 in the presence of GRFT with or without plasma starting at a 1:20 dilution. Only the CI at ID50 was measured, as the highest neutralization with both plasmas was below 80%. GRFT and BB10 acted synergistically to neutralize all 4 viruses tested, with CI values ranging from 0.2 to 0.7. In contrast, GRFT and BB34 synergized to neutralize only CAP239.G3J, with a CI of 0.4, while there was antagonism for QH0692.42 (Table 4).
Table 4.
Synergy between GRFT and HIV-1-positive plasma for neutralization of HIV-1
| HIV-1 pseudovirus envelope | BB10 |
BB34 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single |
Combined |
CIa | Single |
Combined |
CIa | |||||
| GRFT IC50 (nM) | BB10 ID50 | GRFT IC50 (nM) | BB10 ID50 | GRFT IC50 (nM) | BB34 ID50 | GRFT IC50 (nM) | BB34 ID50 | |||
| CAP239.G3J | 0.3 | 0.009 | 0.06 | 0.0005 | 0.2 | 0.3 | 0.03 | 0.1 | 0.0008 | 0.4 |
| COT6.15 | 1.4 | 0.03 | 0.7 | 0.005 | 0.7 | 1.4 | 0.0007 | 1.4 | 0.0007 | 1.1 |
| COT9.6 | 0.7 | 0.01 | 0.3 | 0.003 | 0.7 | 0.6 | 0.03 | 0.3 | 0.02 | 1.2 |
| QH0692.42 | 0.07 | 0.04 | 0.05 | 0.0003 | 0.7 | 0.1 | 0.009 | 0.2 | 0.002 | 2.2 |
A CI of 0.3 to 0.7 indicates synergism, 0.7 to 0.85 indicates moderate synergism, 0.85 to 0.9 indicates slight synergism, and 0.9 to 1.1 indicates an additive effect. Cases where synergism occurred are in boldface.
CAP239.G3J showed synergy with both heterologous plasmas, so we examined GRFT in combination with the autologous serum. However, we observed no synergism between the lectin and the serum (data not shown), consistent with the observation that it did not contain anti-CD4bs antibodies (John R. Mascola, unpublished data). In conclusion, these data show that GRFT can increase HIV-1 sensitivity to plasma containing CD4bs antibodies.
GRFT inhibited sCD4-induced 17b binding and blocked infection via both CCR5 and CXCR4.
Given that GRFT inhibits HIV infection by binding glycans and blocking receptor engagement, we asked how the lectin might promote virus binding to the CD4 receptor while inhibiting viral infection. We hypothesized that GRFT inhibits HIV infection by blocking steps that follow the CD4 receptor binding event, such as the coreceptor binding step. To investigate this possibility, we used the HIV-2 pseudovirus 7312A, which, following incubation with soluble CD4, becomes sensitive to the 17b MAb that binds to CD4i epitopes (15). First, we established that 7312A sensitivity to GRFT was similar to that of HIV-1 by carrying out a neutralization assay in TZM-bl cells. GRFT inhibited the virus with an IC50 of 4 nM, which is comparable to what we obtained with HIV-1 (1). We incubated 7312A with GRFT and then with sCD4 prior to capture with 17b. We also reversed the order, i.e., we incubated the virus with sCD4 first before incubation with GRFT prior to capture on the ELISA plate. As shown in Fig. 6A and B, GRFT reduced the amount of virus captured with 17b in the presence of sCD4 irrespective of when sCD4 was added, suggesting that GRFT inhibits the interaction of 17b with the coreceptor binding site.
Fig. 6.
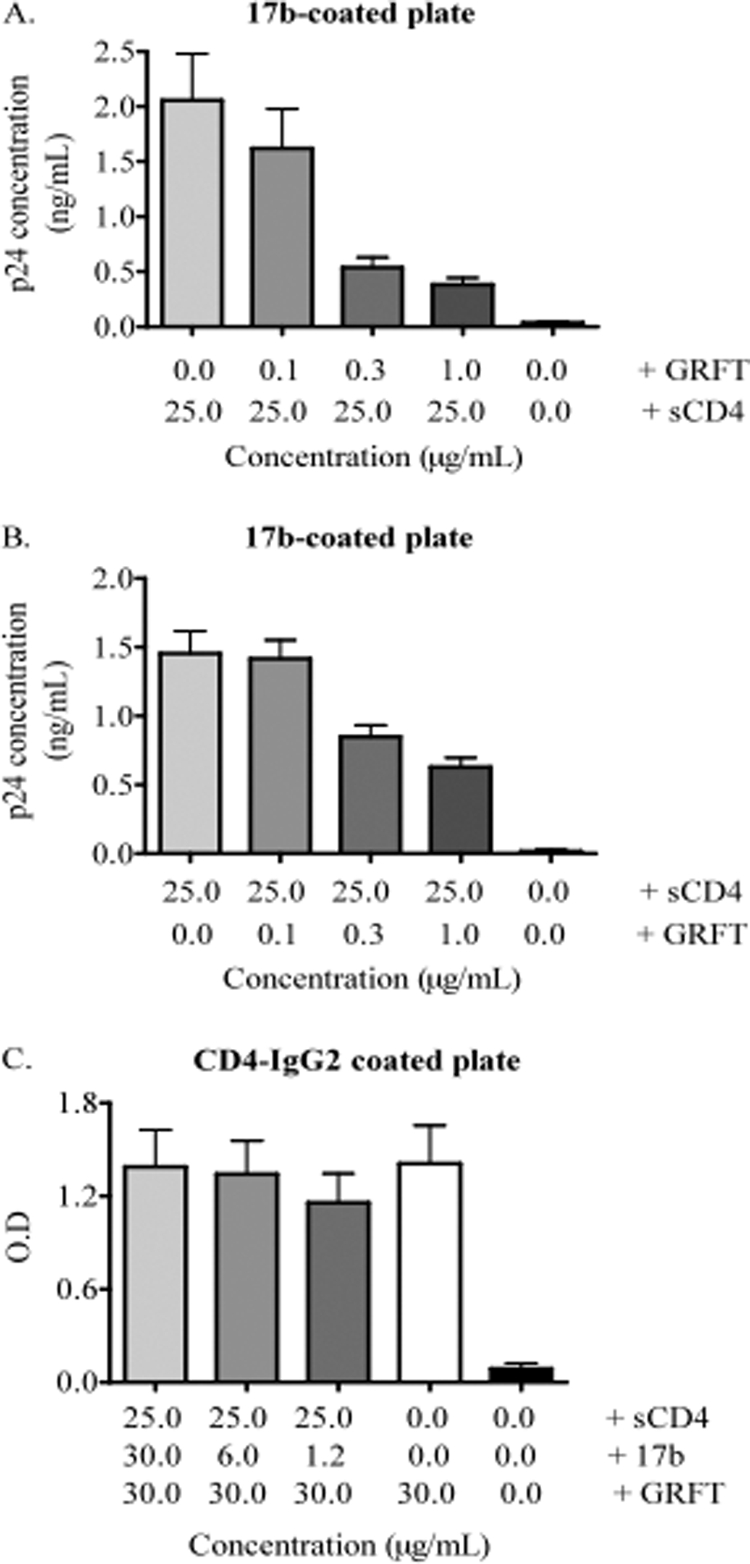
GRFT inhibition of 17b MAb binding to the CD4i epitope. (A) The HIV-2 pseudovirus 7312A was first incubated with different concentrations of GRFT and then with 25 μg/ml sCD4 prior to capture on a 17b-coated plate. The well containing the immobilized 17b only (0 μg/ml of GRFT and sCD4) is the experimental control well. The amount of captured virus was measured by p24 ELISA. The bars represent the means and SD of three different experiments. (B) Same experiment as in panel A, except that 7312A was first incubated with 25 μg/ml of sCD4 before incubation with different concentrations of GRFT. (C) The HIV-2 pseudovirus 7312A was captured with CD4-IgG2 and then incubated with sCD4. This was followed by sequential addition of 17b, GRFT, and anti-GRFT antibody to the captured virus. The white bar (the well containing the virus and GRFT only) is the positive control, while the black bar (the well containing the virus only) is the negative control. The amount of GRFT bound to the virus was measured by measuring the optical density (O.D.) at 450 nM after the addition of horseradish peroxidase and the substrate. The bars represent the means and SD of three different experiments.
To determine if 17b interfered with GRFT binding, we added 7312A to an ELISA plate coated with CD4-IgG2 and then sequentially added sCD4, 17b, and GRFT to the plate before adding rabbit anti-GRFT polyclonal antibodies. Figure 6C shows that the increase in the concentration of 17b had no effect on GRFT binding to the virus. Similarly, GRFT binding to an ELISA plate coated with monomeric gp120 did not reduce 17b binding (data not shown), confirming that GRFT does not interfere with 17b binding. Taken together, these data indicate that GRFT and 17b do not occlude each other's binding sites, i.e., there is no steric obstruction between the two compounds.
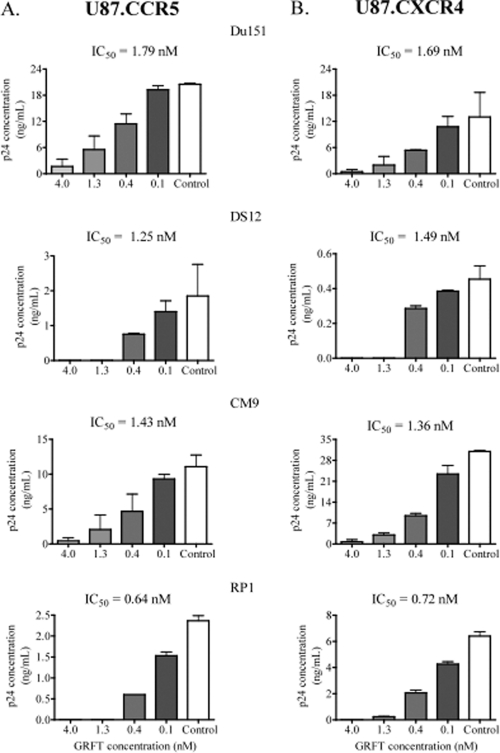
Given the possibility that GRFT interferes with coreceptor binding to HIV-1, we analyzed whether lectin inhibition of the virus was coreceptor specific. U87 cells expressing either the CCR5 or CXCR4 coreceptor were infected with the dual-tropic infectious HIV-1 Du151, DS12, CM9, and RP1 in the presence of GRFT. As shown in Fig. 7, GRFT inhibited infection in both cell lines with similar IC50s regardless of which coreceptor the virus used more efficiently.
Fig. 7.
GRFT inhibited HIV-1 infection in U87-CCR5 and U87-CXCR4 cells. HIV-1 subtype C Du151, DS12, CM9, and RP1 were treated with different concentrations of GRFT before infection of U87-CCR5 (A) and U87-CXCR4 (B) cells. The data are shown as the averages plus standard deviations of three independent experiments. Untreated virus is shown in white (positive control). The IC50s of GRFT are indicated above each graph.
Since different classes of entry inhibitors have been shown to act synergistically to inhibit HIV-1 infection (43, 44, 53, 65), we next determined whether the anti-CCR5 antibody PRO140 synergized with GRFT to inhibit HIV-1 infection. Six pseudoviruses were tested at a GRFT and PRO140 molar ratio of 1 to 50. As shown in Table 5, four viruses showed moderate synergy between GRFT and PRO140 of 0.6 to 0.7 at IC80. This suggested that while GRFT and the anti-CCR5 MAb PRO140 can synergize to inhibit HIV-1 infection, this effect requires high concentrations of both compounds. In addition, since we previously showed that GRFT enhanced QH0692.42 and COT6.15 binding to CD4IgG2 (Fig. 2), we investigated whether the lectin synergized with sCD4 to neutralize these viruses. We found that there was synergism between GRFT and sCD4 for the neutralization of QH0692.42 with CIs of 0.7 and 0.6 at IC50 and IC80, respectively.
DISCUSSION
In this study, we have shown that GRFT enhanced HIV-1 binding to the CD4bs MAbs b12 and b6 and the CD4 surrogate CD4-IgG2. The observed enhancement was specific to GRFT, as no effect was seen with CV-N, another mannose-binding lectin. The enhancement of binding with GRFT was not due to lectin cross-linking of viruses, as HIV-1 binding to gp41- and V3-specific MAbs was not affected. This enhanced binding resulted in moderate synergistic neutralization between b12 and GRFT for some viruses. The high-mannose glycan at position 386 on gp120 was implicated in both the enhancement and synergism between GRFT and b12, probably because the glycan shields the CD4bs (18). Lastly, in this study, we also showed that GRFT inhibits CCR5- and CXCR4-mediated HIV-1 infection with similar potencies and that the lectin can synergize with plasma from HIV-positive individuals and with the anti-CCR5 inhibitor PRO140 to neutralize HIV-1.
GRFT enhancement of HIV-1 binding was higher for subtype B than for subtype C viruses. Glycosylation differences in the envelopes of the two subtypes may explain this (60), although a previous study suggested that these differences had no impact on neutralization by GRFT (1). Another possible reason is that subtype B viruses show higher affinity for b12 than subtype C. Indeed, subtype B viruses are neutralized by b12 and b6 more frequently and more potently than subtype C viruses (5). However, this is unlikely to be the only reason, as PVO.4, which is resistant to b12, showed the highest levels of b12 binding in the presence of GRFT. Clearly, a larger number of viruses from both subtypes should be tested to determine whether substantial differences in GRFT-mediated enhancement of b12 and b6 binding exist.
In general, b6 binding was higher than b12 binding, suggesting that the b6 epitope may be more affected by the GRFT-mediated exposure of the CD4bs. GRFT also increased binding of CD4-IgG2, although the enhancement was lower than for the anti-CD4bs antibodies of the 2 viruses tested. This may be due to the fact that the CD4 receptor binds further away from glycan 386 or because it is simply less affected by GRFT binding to gp120. However, the use of antibodies and the CD4 mimetic provides compelling evidence that GRFT exposes the entire CD4bs and not just the binding sites of the antibodies.
In 2 of the 4 viruses tested, GRFT-mediated enhanced binding to b12 resulted in synergistic neutralization between GRFT and b12. The individual virus sensitivity to GRFT and b12 neutralization, as well as the level of GRFT-mediated enhancement of binding to b12, is likely to be an important determining factor for neutralization synergy to occur. Thus, QH0692.42 and COT6.15, the 2 viruses in which synergy was observed, showed the highest levels of enhancement, and both were sensitive to GRFT and b12 neutralization. However, for COT6.15, the ratio of b12 to GRFT used to achieve synergism was very high, probably because high levels of b12 were required for COT6.15 neutralization. The lack of synergy for COT9.6 may be explained by the comparatively low levels of enhancement of b12 binding in the presence of GRFT. The Du151.2 virus did not show synergism despite being sensitive to b12 and GRFT, and its enhancement was similar to that of COT6.15, suggesting that other factors were involved; for example, this virus was the only one that lacked the 339 glycan. Two viruses were not sensitive to b12, so synergy could not be assessed; PVO.4 resistance was probably due to trimer shielding, as the virus had all the required b12 contact residues, while in CAP239.G3J, resistance to b12 was due to sequence variation at these sites (61). Although no synergism was noted between GRFT and b6, as expected given that it is a nonneutralizing antibody, the lectin acted synergistically with sCD4 to neutralize QH0692.42. This suggested that, like the enhancement of binding, GRFT-mediated synergy involved more than just the b12 binding site. Furthermore, synergy between GRFT and HIV-positive plasma BB10, which contains anti-CD4bs antibodies (25), for all the viruses tested is consistent with GRFT exposure of the CD4bs. However, the lack of synergistic interaction between the lectin and BB34 for 3 of these viruses is in agreement with the fact that the plasma contains antibodies that target the MPER (25). This supports the data in Fig. 4 that show GRFT does not enhance HIV-1 binding to 4E10.
We also observed synergism at IC80 between GRFT and the CCR5 inhibitor PRO140 for the neutralization of HIV-1 in 4 of the 6 viruses tested. However, the mechanism of this interaction is most probably unrelated to that of GRFT/b12, since the PRO140 MAb binds CCR5 on the cell surface, thereby blocking coreceptor interactions, while the b12 MAb binds gp120, blocking the initial association with CD4. Synergy between PRO140 and other CCR5 inhibitors has been noted previously with CI values between 0.38 and 0.61 (43). This is similar to what is reported in this study, which for GRFT in combination with PRO140, b12, or sCD4 was between 0.6 and 0.7, while for BB10 plasma it was 0.2 to 0.7. Comparison to CIs that have been published for other HIV-1 inhibitors, including a fusion inhibitor, such as AMD3100 and T20 (0.03) and PRO542 and T20 (0.14), suggests that the synergistic interactions we observed with GRFT were moderate (44, 53).
The high-mannose glycan at position 386 is located within the CD4bs (29, 32, 48), and deletion of this glycan has been shown to increase HIV-1 neutralization sensitivity to b12 (24, 52). It is possible that the mechanism of GRFT-mediated enhancement of b12 binding and synergy is the result of GRFT binding to 386 and shifting the glycan to increase accessibility to the CD4bs. Removal of the glycan resulted in a decrease in b12 binding and also decreased synergy between GRFT and b12. Deletion of the 386 glycan probably impacted GRFT binding to gp120, as was suggested by QH0692.42 (Table 3). Duenas-Decamp et al. (17) showed that the 386 glycosylation site requires arginine at position 373 to modulate HIV-1 resistance to b12. However, given that none of the viruses we tested had arginine 373, this residue is most probably not important for the GRFT-mediated enhancement of HIV-1 binding to b12.
CV-N, like GRFT, binds high-mannose residues, and the glycan at position 386 has also been implicated in its binding site (3). However, the lectin did not show the same effects as GRFT, suggesting that GRFT interacts with high-mannose glycans on gp120 somewhat differently than CV-N. Perhaps the arrangement of the 6 glycan binding sites on GRFT may allow greater cross-linking of glycans on gp120 than the 4 binding sites of CV-N. Recent structural studies on monomeric GRFT have indicated that such intraspike cross-linking is important for the antiviral activity of GRFT (42). This being said, it is clear that the GRFT enhancement is not due to cross-linking between viral particles, as binding to the gp41 and anti-V3 MAbs was not enhanced. The competition between GRFT and 17b for binding to HIV-2 7312A in the presence of sCD4 suggested that GRFT may inhibit HIV-1 binding to the coreceptor after CD4 receptor engagement (Fig. 6A and B). GRFT is likely to achieve this regardless of whether it binds before or after the CD4 receptor binding to gp120, although the effect is more pronounced when GRFT exposure precedes CD4 binding. However, the lack of steric obstruction between 17b and GRFT suggested that GRFT interference with the coreceptor binding site may not involve direct steric hindrance. We speculate that dimeric GRFT binding to multiple glycans on gp120 interferes with the structural rearrangement induced by CD4 binding necessary to form the CD4i epitope. Lastly, the similarity in the potencies of GRFT neutralization of CCR5- and CXCR4-mediated HIV-1 infection is likely to be related to the fact that the lectin inhibits the virus by targeting the viral envelope and not cellular receptors.
The CD4-binding site is a very important target for HIV-1 neutralizing antibodies, since the site is conserved among different HIV-1 subtypes (14, 29, 61). This is supported by the fact that b12, one of the few HIV-1 broadly neutralizing antibodies, targets this epitope (26). More recently, highly potent anti-CD4bs MAbs, VRC01, VRC02, and VRC03, which neutralize a much broader spectrum of isolates from diverse genetic subtypes, have been isolated (57). The vital role played by the CD4 receptor in the HIV-1 life cycle (14, 28) suggests that antibodies to this site could block replication in vivo, as has been shown in macaque studies (49). Given the unique mode of action of lectins and the fact that most are not toxic in vitro or in animal models, it has been suggested that these compounds represent a novel approach for intravenous targeting of HIV (2). As has been shown, the ability of GRFT to expose the CD4bs may offer a way of making HIV-1 more susceptible to neutralization by anti-CD4bs antibodies, which are found in some HIV-positive patients (25). The study reported here increases our understanding of the interaction of GRFT with HIV-1 gp120 and also suggests a new way of increasing the exposure of the CD4bs.
ACKNOWLEDGMENTS
This work was funded by a Biofisa grant from NEPAD, the South African AIDS Vaccine Initiative (SAAVI), and a training fellowship to K.B.A. from Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH (AITRP grant D43TW00231). This research was also supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (B.R.O. and J.B.M.).
We thank Progenics Pharmaceuticals, Inc., and Dennis Burton for providing some of the reagents used in this study.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Alexandre K. B., et al. 2010. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology 402:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balzarini J. 2005. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect. Dis. 5:726–731 [DOI] [PubMed] [Google Scholar]
- 3. Balzarini J., et al. 2006. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J. Virol. 80:8411–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbas C. F., III, et al. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. U. S. A. 89:9339–9343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Binley J. M., et al. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232–13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyd M. R., et al. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchacher A., et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses 10:359–369 [DOI] [PubMed] [Google Scholar]
- 8. Burrer R., Haessig-Einius S., Aubertin A. M., Moog C. 2005. Neutralizing as well as non-neutralizing polyclonal immunoglobulin (Ig)G from infected patients capture HIV-1 via antibodies directed against the principal immunodominant domain of gp41. Virology 333:102–113 [DOI] [PubMed] [Google Scholar]
- 9. Calarese D. A., et al. 2005. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. U. S. A. 102:13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavacini L., Posner M. 2004. Native HIV type 1 virion surface structures: relationships between antibody binding and neutralization or lessons from the viral capture assay. AIDS Res. Hum. Retroviruses 20:435–441 [DOI] [PubMed] [Google Scholar]
- 11. Chou T. C., Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27–55 [DOI] [PubMed] [Google Scholar]
- 12. Cilliers T., et al. 2003. The CCR5 and CXCR4 coreceptors are both used by human immunodeficiency virus type 1 primary isolates from subtype C. J. Virol. 77:4449–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coetzer M., et al. 2007. Longitudinal analysis of HIV type 1 subtype C envelope sequences from South Africa. AIDS Res. Hum. Retroviruses 23:316–321 [DOI] [PubMed] [Google Scholar]
- 14. Dalgleish A. G., et al. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767 [DOI] [PubMed] [Google Scholar]
- 15. Decker J. M., et al. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 201:1407–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doores K. J., et al. 2010. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. U. S. A. 107:13800–13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duenas-Decamp M. J., Peters P., Burton D., Clapham P. R. 2008. Natural resistance of human immunodeficiency virus type 1 to the CD4bs antibody b12 conferred by a glycan and an arginine residue close to the CD4 binding loop. J. Virol. 82:5807–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunfee R. L., et al. 2007. Loss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementia. Virology 367:222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geijtenbeek T. B., Gringhuis S. I. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9:465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Go E. P., et al. 2009. Glycosylation site-specific analysis of clade C HIV-1 envelope proteins. J. Proteome Res. 8:4231–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gray E. S., Meyers T., Gray G., Montefiori D. C., Morris L. 2006. Insensitivity of paediatric HIV-1 subtype C viruses to broadly neutralising monoclonal antibodies raised against subtype B. PLoS Med. 3:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gray E. S., et al. Isolation of a monoclonal antibody targeting the alpha-2 helix of gp120 representing the initial autologous neutralizing antibody response in an HIV-1 subtype C infected individual. J. Virol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gray E. S., et al. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gray E. S., Moore P. L., Pantophlet R. A., Morris L. 2007. N-linked glycan modifications in gp120 of human immunodeficiency virus type 1 subtype C render partial sensitivity to 2G12 antibody neutralization. J. Virol. 81:10769–10776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray E. S., et al. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haigwood N. L. 2006. Insights into neutralizing antibodies and HIV envelope. Curr. Opin. HIV AIDS. 1:301–308 [DOI] [PubMed] [Google Scholar]
- 27. Johnson V. A., Byington R. E. 1990. Quantitative assays for virus infectivity, p. 71–76 In Aldovini A., Walker B. D. (ed.), Techniques in HIV research. Stockton Press, New York, NY [Google Scholar]
- 28. Klatzmann D., et al. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768 [DOI] [PubMed] [Google Scholar]
- 29. Kwong P. D., et al. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lasky L. A., et al. 1986. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science 233:209–212 [DOI] [PubMed] [Google Scholar]
- 31. Leaman D. P., Kinkead H., Zwick M. B. 2010. In-solution virus capture assay helps deconstruct heterogeneous antibody recognition of human immunodeficiency virus type 1. J. Virol. 84:3382–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leonard C. K., et al. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373–10382 [PubMed] [Google Scholar]
- 33. Li M., et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y., Luo L., Rasool N., Kang C. Y. 1993. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J. Virol. 67:584–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin G., et al. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu F., et al. 2005. Human single-chain antibodies inhibit replication of human immunodeficiency virus type 1 (HIV-1). AIDS Res. Hum. Retroviruses 21:876–881 [DOI] [PubMed] [Google Scholar]
- 37. Liu Y., et al. 2004. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J. Virol. 78:4120–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Losman B., et al. 2001. Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoprotein 120 by N-linked oligosaccharides in the V1 region. AIDS Res. Hum. Retroviruses 17:1067–1076 [DOI] [PubMed] [Google Scholar]
- 39. Lue J., et al. 2002. Addition of a single gp120 glycan confers increased binding to dendritic cell-specific ICAM-3-grabbing nonintegrin and neutralization escape to human immunodeficiency virus type 1. J. Virol. 76:10299–10306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Montefiori D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p. 12.11.1–12.11.15 In Coligan J. E., Kruisbeek A. M., Margulies D. H., Shevach E. M., Strober W., Coico R. (ed.), Current protocols in immunology. John Wiley & Sons, New York, NY: [DOI] [PubMed] [Google Scholar]
- 41. Mori T., et al. 2005. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 280:9345–9353 [DOI] [PubMed] [Google Scholar]
- 42. Moulaei T., et al. 2010. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure 18:1104–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murga J. D., Franti M., Pevear D. C., Maddon P. J., Olson W. C. 2006. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 50:3289–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagashima K. A., et al. 2001. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J. Infect. Dis. 183:1121–1125 [DOI] [PubMed] [Google Scholar]
- 45. Nyambi P. N., et al. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 72:9384–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Keefe B. R., et al. 2003. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 47:2518–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Keefe B. R., et al. 2009. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. U. S. A. 106:6099–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pantophlet R., et al. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77:642–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parren P. W., et al. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poignard P., et al. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roben P., et al. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanders R. W., et al. 2008. The carbohydrate at asparagine 386 on HIV-1 gp120 is not essential for protein folding and function but is involved in immune evasion. Retrovirology 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tremblay C. L., Kollmann C., Giguel F., Chou T. C., Hirsch M. S. 2000. Strong in vitro synergy between the fusion inhibitor T-20 and the CXCR4 blocker AMD-3100. J. Acquir. Immune Defic. Syndr. 25:99–102 [DOI] [PubMed] [Google Scholar]
- 54. van Gils M. J., et al. 2010. Rapid escape from preserved cross-reactive neutralizing humoral immunity without loss of viral fitness in HIV-1-infected progressors and long-term nonprogressors. J. Virol. 84:3576–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei X., et al. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312 [DOI] [PubMed] [Google Scholar]
- 56. Williamson C., et al. 2003. Characterization and selection of HIV-1 subtype C isolates for use in vaccine development. AIDS Res. Hum. Retroviruses 19:133–144 [DOI] [PubMed] [Google Scholar]
- 57. Wu X., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wyatt R., Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888 [DOI] [PubMed] [Google Scholar]
- 59. Xu W., et al. 2001. Potent neutralization of primary human immunodeficiency virus clade C isolates with a synergistic combination of human monoclonal antibodies raised against clade B. J. Hum. Virol. 4:55–61 [PubMed] [Google Scholar]
- 60. Zhang M., et al. 2004. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 14:1229–1246 [DOI] [PubMed] [Google Scholar]
- 61. Zhou T., et al. 2007. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445:732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu X., Borchers C., Bienstock R. J., Tomer K. B. 2000. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry 39:11194–11204 [DOI] [PubMed] [Google Scholar]
- 63. Ziółkowska N. E., et al. 2006. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 14:1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ziółkowska N. E., Wlodawer A. 2006. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 53:617–626 [PubMed] [Google Scholar]
- 65. Zwick M. B., et al. 2005. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J. Virol. 79:1252–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zwick M. B., et al. 2003. Molecular features of the broadly neutralizing immunoglobulin G1 b12 required for recognition of human immunodeficiency virus type 1 gp120. J. Virol. 77:5863–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zwick M. B., et al. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198–12208 [DOI] [PMC free article] [PubMed] [Google Scholar]