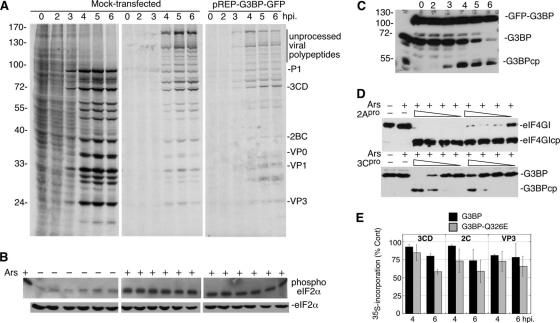
Fig. 2.
Expression of cleavage-resistant G3BP does not cause resistance to Ars stress. (A) HeLa cells expressing hamster pREP-G3BP-GFP were infected, stressed, and pulse-labeled with Tran35S-label and analyzed as described in the legend to Fig. 1A. The migration positions of molecular mass standards (in kilodaltons) are indicated to the left of the gel. (B) Immunoblot analysis of lysates for total and phosphorylated eIF2α. (C) Immunoblot analysis for G3BP showing the cleavage of endogenous G3BP but not G3BP-GFP. (D) HeLa S10 lysates were treated with a constant concentration of Ars (1 mM) plus various concentrations (1 μg to 10 ng) of recombinant viral 2Apro or 3Cpro to determine the effects of Ars treatment on proteinase activity. The height of the triangle above the lanes indicates the relative amount of 2Apro or 3Cpro. 2A and 3C proteinase activities were monitored by the cleavage of eIF4GI and G3BP, respectively. (E) 293T cells transfected with control DNA or plasmids expressing His-G3BP or His-G3BPQ326E were infected with PV, translating proteins were pulse-labeled at 4 or 6 hpi and analyzed by SDS-PAGE/autoradiography and densitometry of the indicated viral polypeptide bands. Data are expressed as percent incorporation relative to untransfected control cells (% Cont).