Figure 10.
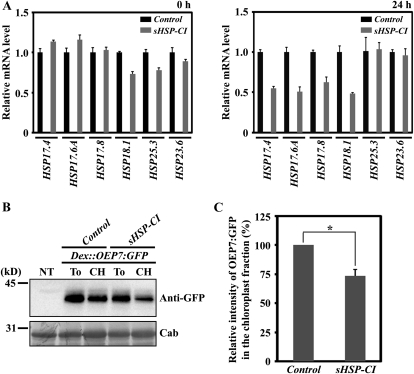
Suppression of CI cytosolic sHSP transcript levels decreases the chloroplast-targeting efficiency of OEP7:GFP in protoplasts. A, Real-time quantitative RT-PCR of various sHSP transcript levels in protoplasts. Protoplasts were transformed with the sHSP-CI amiRNA construct or control amiRNA vector. Total RNA extracted from the transformed protoplasts at 0 and 24 h after transformation was subjected to quantitative RT-PCR analysis using gene-specific primers. Quantitative RT-PCR was performed at 95°C for 15 s and 60°C for 1 min for 40 cycles. Actin8 was used as an internal control for the quantitative RT-PCR. Error bars represent se (n = 3). B and C, Effect of sHSP-CI amiRNA on the OEP7:GFP targeting to chloroplasts. B, Dex::OEP7:GFP was cotransformed into protoplasts together with sHSP-CI amiRNA or the control amiRNA vector. Transformed protoplasts were treated with dexamethasone 24 h after transformation and incubated for an additional 12 h. Subsequently, protoplasts were divided into two fractions: one fraction was used to purify chloroplasts by Percoll gradients, and the second fraction was used for total protein extracts. Proteins from the purified chloroplasts together with total protein extracts from the protoplasts were analyzed by western blotting using anti-GFP antibody. NT, Nontransformed protoplasts; To, total protein extracts; CH, protein extracts from purified chloroplasts; Cab, chlorophyll a/b-binding protein, used as a loading control. C, To quantify the targeting efficiency, the intensity of OEP7:GFP in the chloroplast fractions was measured and is presented as a relative value to that of control amiRNA vector. The asterisk denotes a statistically significant difference compared with control amiRNA vector (P < 0.01; n = 3). Error bars represent se (n = 3).