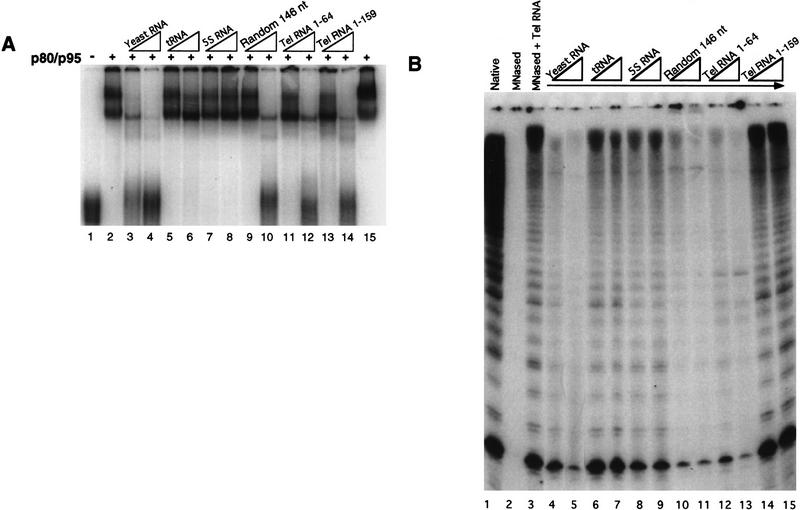
Figure 3.
The specificity of telomerase RNA association with recombinant p80/p95 complex or with purified, endogenous Tetrahymena telomerase proteins is similar in vitro. (A) The p80/p95 complex was used in a mobility shift assay at a concentration of 100 nm with 40 nm radiolabeled telomerase RNA. (Lane 1) Probe in the absence of proteins. The mobility shift caused by p80/p95 in the absence of competitors is shown in lane 2. Because molar equivalents could not be determined for total yeast RNA, unlabeled competitor RNAs were added into the binding reaction at 100 ng and 1 μg (5- and 50-fold the mass amount of probe): (lanes 3,4) total yeast RNA; (lanes 5,6) tRNA; (lanes 7,8) 5S RNA; (lanes 9,10) a random 146 nucleotide transcript (see Materials and Methods); (lanes 11,12) a 64 nucleotide region of the T. thermophila telomerase RNA; (lanes 13,14) 159-nucleotide full-length T. thermophila telomerase RNA. All reactions except that in lane 15 were conducted with a 5-min heating step at 37°C with 5 mm EDTA (conditions for reconstitution of telomerase activity with recombinant RNA and endogenous proteins). The lane 15 reaction has the EDTA step omitted to show that these conditions do not affect the nature of the shift. (B) Native telomerase was reconstituted with in vitro-transcribed telomerase RNA in the presence of the same RNA competitors as in A. Native telomerase activity on d(G4T2)3 is shown in lane 1. Lanes 4–15 have the indicated RNAs added during the reconstitution step. These RNAs were added at 400 ng and 1 μg (two- and fivefold the level of wild-type telomerase RNA). Lanes 14 and 15 have 400 ng and 1 μg of additional wild-type telomerase RNA to show that excess RNA per se does not inhibit the reaction. Some of the activity in lanes 12 and 13 is attributable to the ability of the competitor RNA to reconstitute an active enzyme (data not shown).