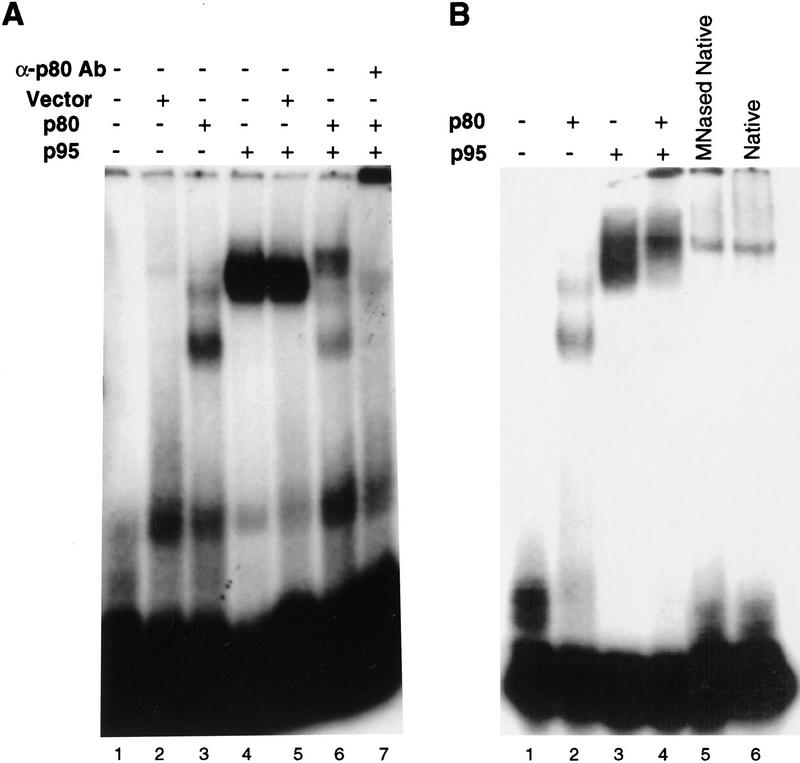
Figure 5.
The p80/p95 complex binds single-stranded, G-rich telomeric sequence DNA. (A) Approximately 2 μg of total protein from E. coli lysates of the empty vector, pRSET (lane 2), p80 (lane 3), or p95 (lane 4), were mixed with radiolabeled d(G4T2)3 at 20 nm final concentration in a mobility shift assay. For combined extract shifts (as indicated in lanes 5 and 6), 2 μg of each extract was used. Extracts were comparable to those shown in Fig. 1C, with similar amounts of p80 or p95 per microgram of extract protein. Assays were conducted in the presence of a mix of single-stranded 18-nucleotide competitor DNAs and double-stranded poly[d(I–C)] (see Materials and Methods). The p80/p95 complex mobility shift (lane 6) was supershifted to the well by the presence of p80 antibodies (lane 7). (B) Purified proteins at a concentration of 100 nm were mixed as indicated with radiolabeled d(G4T2)3 at a concentration of 20 nm. All reactions contained the same mixture of competitor DNAs as in A. MNase-treated telomerase with degraded endogenous RNA (lane 5) and native telomerase (lane 6) are purified fractions containing substantially less protein.