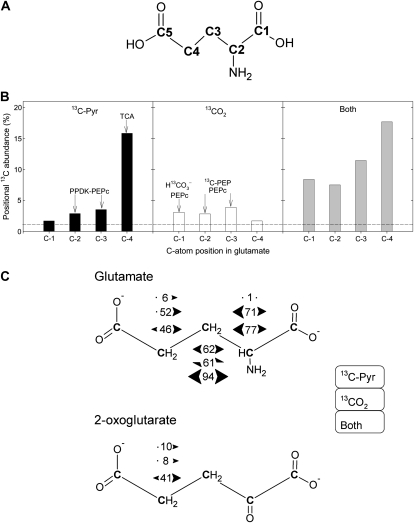
Figure 3.
Positional 13C-NMR signals in Glu, after 13C-3-pyruvate and/or 13CO2 labeling of illuminated cocklebur leaves for 3 h, under 400 μmol mol−1 CO2, 21% O2, 23.5°C, and 300 μmol m−2 s−1 PAR. A, C-atom numbering in Glu. B, Positional 13C abundance (in % 13C) in Glu (redrawn from Fig. 2). The natural 13C abundance (1.1%) is indicated with the horizontal dashed line. The major origin of 13C labeling in each position is indicated with arrows, as discussed in the main text. C, {13C-13C} spin-spin interactions in Glu. For a given labeled position, numbers between arrows indicate the percentage of C atoms having 13C labeling in the neighbor C atoms. Bidirectional arrows indicate that two {13C-13C} interactions were obtained, demonstrating there were two interacting, labeled neighbor C. For example, in C-3 Glu after pyruvate labeling, the percentage of 13C in C-3 that was accompanied by 13C in C-2 and C-4 was 62%. In 2-oxoglutarate, {13C-13C} spin-spin interactions were only visible (distinguishable from the background) in C-4 because of the larger 13C enrichment in that position. The value of 1% (Glu C-2 upon 13C-pyruvate labeling) is arbitrary and corresponds to the natural interaction (13C natural abundance of 1.1%). Bidirectional half arrows indicate that a single {13C-13C} interaction occurred, on one side only of the C-atom position considered. From top to bottom, interactions values indicated here are that observed after 13C-pyruvate labeling, 13CO2 labeling, and after double labeling, in that order.