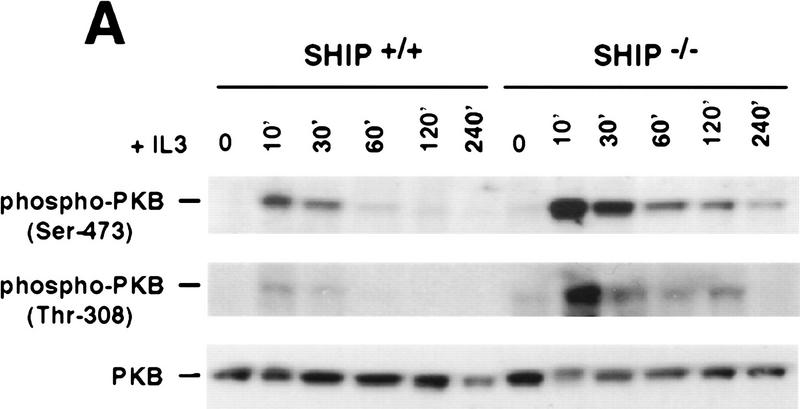
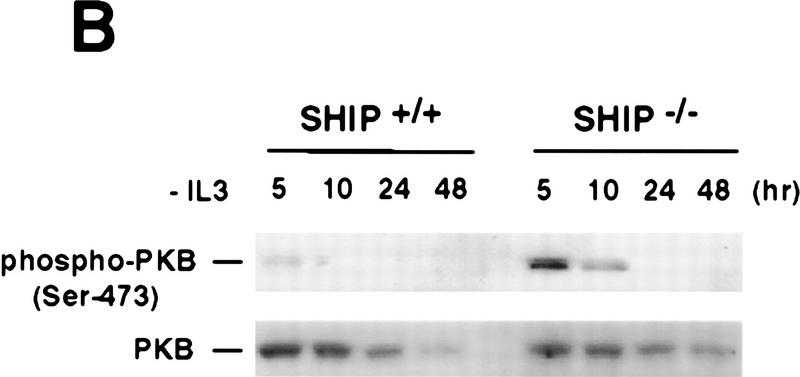
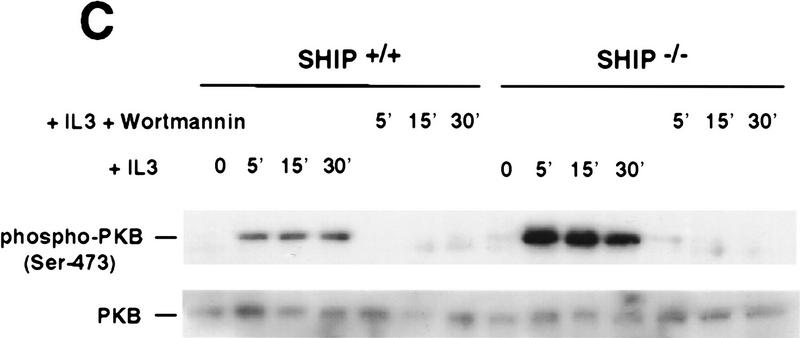
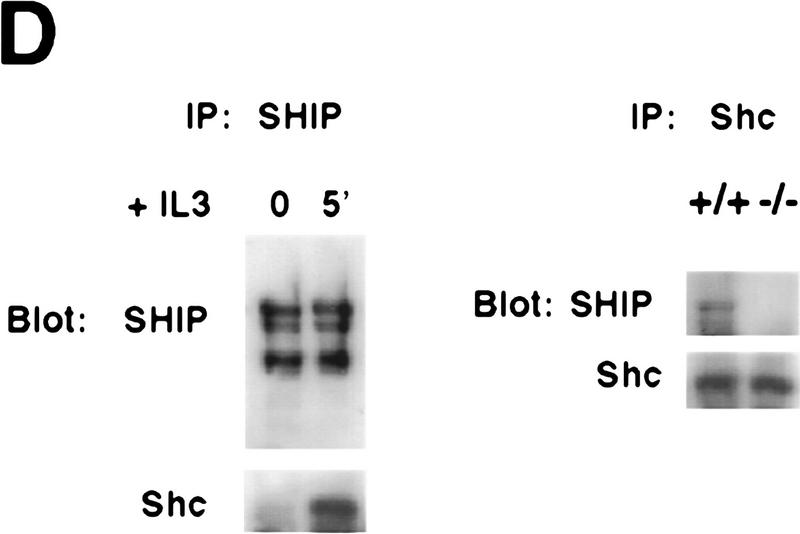
Figure 3.
Negative regulation of PKB phosphorylation by SHIP. (A) Increased PKB phosphorylation in SHIP-deficient BMMCs following stimulation with IL3. Because SHIP−/− BMMCs have elevated baseline levels of activated PKB, cells were starved of IL3 for 24 hr prior to induction. At this time point, no PKB phosphorylation was detectable in SHIP+/+ and SHIP−/− cells. Serum-starved BMMCs were stimulated with 5 ng/ml IL3 for the indicated time periods. Total cell lysates were analyzed for activated PKB by Western blot using antibodies specific for Ser-473 (top), or Thr-308 (middle), and total PKB protein (bottom). The middle panel was from a different gel in which the same cell extracts were loaded and equal loading was confirmed by PKB levels (not shown). (B) Prolonged and increased PKB phosphorylation (Ser-473) in SHIP-deficient BMMCs following IL3 withdrawal. Time points after IL3 withdrawal are indicated. Note the enhanced baseline of PKB phosphorylation in SHIP−/− BMMCs at 5 hr after IL3 starvation. This level of phospho-Ser-473 is similar to that in SHIP+/+ BMMCs cultured in IL3 (not shown). (A,B) One result is representative of five experiments. (C) SHIP-regulated PKB activation is PI3′K-dependent. Western blot analysis of cell lysates from serum-starved (A) SHIP+/+ and SHIP−/− BMMCs treated with IL3 (5 ng/ml) in the presence or absence of Wortmannin (100 nm) for the indicated time periods. (D) SHIP associates with Shc following IL3 stimulation. Serum-starved SHIP+/+ mast cells were stimulated with IL3 (5 ng/ml) for various time periods. SHIP and Shc were immunoprecipitated independently and interactions between SHIP and Shc visualized by Western blot. (C,D) One result is representative of three independent experiments.