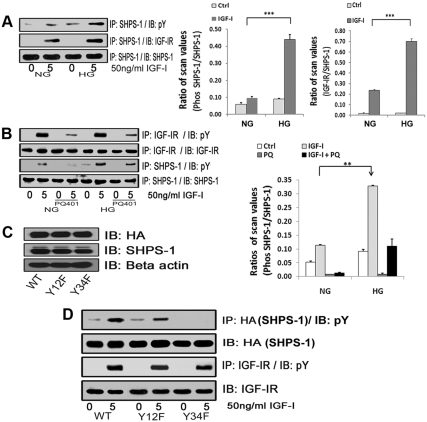
Fig. 1.
Effects of high glucose on IGF-I-stimulated SHPS-1 phosphorylation and IGF-IR/SHPS-1 association. A, Quiescent VSMC cultures were incubated in SFM containing 5 (DMEM-NG) or 25 (DMEM-HG) mm glucose overnight. Cells were stimulated with IGF-I (50 ng/ml) for 5 min. The extent of SHPS-1 tyrosine phosphorylation was determined by immunoprecipitating (IP) cell lysates with an anti-SHPS-1 antibody and then immunoblotting (IB) with an antiphosphotyrosine (pY) antibody (upper panel). Similarly the extent of the SHPS-1 association with IGF-IR was determined by IP using an anti-SHPS-1 antibody and then IB with an anti IGF-IR antibody (middle panel). As a loading control, the cell lysates were immunoprecipitated and immunoblotted with an anti-SHPS-1 antibody (lower panel). The bar graphs show pooled results from at least three independent experiments. The error bars represent mean ± se. ***, P < 0.001 when amount of SHPS-1 phosphorylation or its association with IGF-IR at 5 min in response to IGF-I is compared between DMEM-NG and DMEM-HG cells. B, Quiescent VSMC cultured and maintained in DMEM-NG or DMEM-HG were serum starved and then incubated with or without the IGF-IR tyrosine kinase inhibitor, PQ401 (10 μm) for 1–2 h. IGF-I was added for 5 min. Cell lysates were immunoprecipitated with an anti-IGF-IR antibody (first panel) or with an anti-SHPS-1 antibody (third panel) and immunoblotted for pY. For loading controls, IP and IB of cell lysates were performed with anti-IGF-IR (second panel) or anti-SHPS-1 antibody (fourth panel). The bar graphs show pooled results from at least three independent experiments. Error bars represent mean ± se. **, P < 0.01 when amount of SHPS-1 phosphorylation at 5 min in response to IGF-I is compared between DMEM-NG and DMEM-HG cells. C, VSMC expressing WT SHPS-1 (SHPS1-WT), VSMC expressing Y428F/Y452F mutant (Y12F), and VSMC expressing Y469F/Y495F mutant (Y34F) were serum starved in DMEM-HG and analyzed for recombinant protein expression. Cell lysates were immunoblotted using an anti-HA antibody (top panel), anti-SHPS-1 antibody (middle panel), and anti-β-actin antibodies (bottom panel). D, Confluent cells expressing SHPS1-WT or the SHPS-1-mutants (Y12F and Y34F) were serum starved for 16 h in DMEM-HG and then exposed to IGF-I for 5 min. The extent of HA-tagged SHPS-1 and IGF-IR phosphorylation was determined by IP HA (first panel) or IGF-IR (third panel) and then immunoblotted with an anti-pY antibody. An equal amount of protein from cell lysates from the same experiment was used to immunoblot for total HA (second panel) and total IGF-IR (fourth panel).