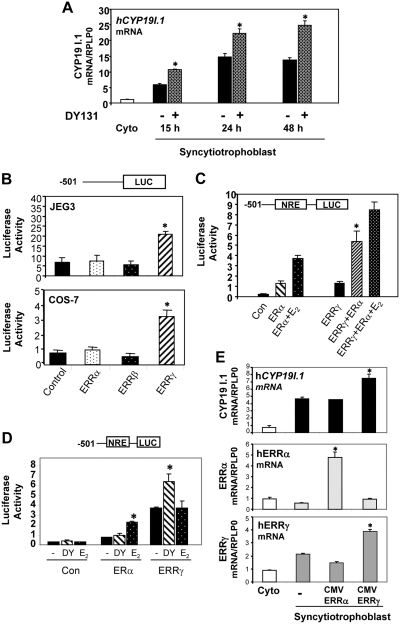
Fig. 2.
Activation or overexpression of ERRγ induces hCYP19I.1 mRNA expression in human trophoblast cells. A, Freshly isolated human cytotrophoblasts were suspended in DMEM with 2% FBS, plated at a density of 2 × 106 cells per dish, and treated with 10 μm of the ERRγ-selective agonist, DY131, or vehicle. RNA was isolated from freshly isolated cytotrophoblasts before (Cyto) or after 15 h, 24 h, or 48 h of culture in DMEM; hCYP19I.1 mRNA was analyzed by qRT-PCR. *, Significantly different (P < 0.05) from vehicle-treated cells. B, COS-7 or JEG-3 cells were transiently cotransfected with a luciferase reporter plasmid containing 501 bp of hCYP19I.1 5′-flanking DNA (−501 hCYP19I.1/Luc) and either ERRα-, ERRβ-, or ERRγ-CMV expression plasmids or an empty expression plasmid as control (Con). Cells also were cotransfected with β-gal expression plasmid to monitor transfection efficiency. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency with β-gal activity. *, Significantly different (P < 0.05) from cells transfected with empty expression vector. C, COS-7 cells were transiently cotransfected with a luciferase reporter plasmid containing 501 bp of hCYP19I.1 5′-flanking DNA (−501 hCYP19I.1/Luc) and either ERα-, or ERRγ-CMV expression plasmids or an empty expression plasmid, as control (Con), and cotransfected with β-gal expression plasmid to monitor transfection efficiency. The cells were treated with 10 nm E2 or vehicle. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments each conducted in triplicate and are expressed as luciferase activity corrected for transfection efficiency by analysis of β-gal activity. *, Significantly different (P < 0.05) from cells transfected with either ERα or ERRγ. D, COS-7 cells were transiently cotransfected with a luciferase reporter plasmid containing 501 bp of hCYP19I.1 5′-flanking DNA (−501 hCYP19I.1/Luc) and either ERα-, or ERRγ-CMV expression plasmids or an empty expression plasmid, as control (Con), and cotransfected with β-gal expression plasmid to monitor transfection efficiency. The cells were treated with 10 μm of the ERRγ-selective agonist, DY131, or 10 nm E2 or vehicle. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency by analysis of β-gal activity. *, Significantly different (P < 0.05) from cells treated with vehicle. E, Freshly isolated human placental cytotrophoblasts plated at a density of 2 × 106 cells per dish were infected with recombinant adenoviruses containing CMV-ERRα or CMV-ERRγ and cultured for 48 h; RNA was isolated and analyzed for expression of hCYP19I.1, ERRα, and ERRγ mRNA by qRT-PCR; RPLP0 was used as the reference. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are compared with expression levels in cytotrophoblasts before culture. *, Significantly different (P < 0.05) from values in cytotrophoblast.