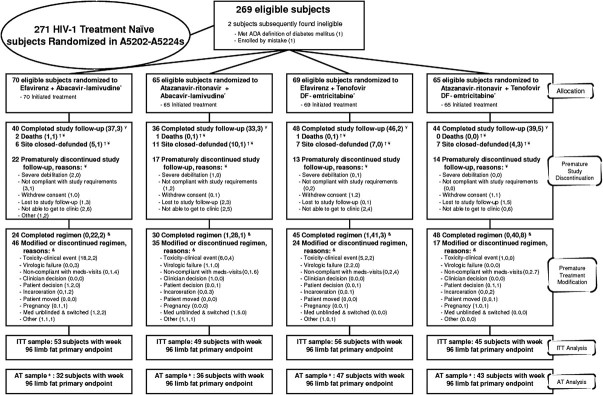
Figure 1.
Details of disposition and outcome of study subjects. Subjects were to remain in follow-up regardless of having modified antiretroviral therapy. Nucleoside reverse-transcriptase inhibitors (NRTIs) were double-blinded through 25 February 2008 for those with screening human immunodeficiency virus (HIV) RNA levels of ≥100,000 copies/mL and until final visits starting 1 July 2009 for those with screening HIV RNA levels <100,000 copies/mL. Reasons for study discontinuation are split into no. of subjects with and no. of subjects without week 96 limb fat primary endpoint. Site closure was censored for premature study and treatment discontinuation. Death was censored for premature study discontinuation and counted as the reason for treatment discontinuation if there was no prior modification. Reasons for the first treatment modification are split into no. of subjects before, no. of subjects after, and no. of subjects without week 96 limb fat primary endpoint. Subjects in the as-treated sample continued to receive randomized treatment through the week 96 whole-body dual-energy X-ray absorptiometry.