In a large group of HIV-infected clinical trial participants with diverse opportunistic infections, blood beta-glucan was a more sensitive noninvasive test for PCP than serum LDH; sensitivity was also higher than that frequently reported for induced sputum examinations.
Abstract
(See the editorial commentary by Morris and Masur, on pages 203–204.)
Background. Improved noninvasive diagnostic tests for Pneumocystis jirovecii pneumonia (PCP) are needed. We evaluated the test characteristics of plasma (1→3)-β-D-glucan (β-glucan) for HIV-related PCP among a large group of patients presenting with diverse opportunistic infections (OIs).
Methods. The study population included all 282 participants in AIDS Clinical Trials Group A5164, a study of early versus deferred antiretroviral therapy in conjunction with initial therapy of acute OIs. Baseline plasma samples were assayed for β-glucan, with standard assay reference values defining ≥80 pg/mL as positive. Before this analysis, diagnosis of PCP was independently adjudicated by 2 study investigators after reviewing reports from study sites.
Results. A total of 252 persons had a β-glucan result that could be analyzed, 173 (69%) of whom had received a diagnosis of PCP. Median β-glucan with PCP was 408 pg/mL (interquartile range [IQR], 209–500 pg/mL), compared with 37 pg/mL (IQR, 31–235 pg/mL) without PCP (P < .001). The sensitivity of β-glucan dichotomized at 80 pg/mL for the diagnosis of PCP was 92% (95% confidence interval [CI], 87%–96%), and the specificity was 65% (95% CI, 53%–75%); positive and negative predictive values were 85% (95% CI, 79%–90%) and 80% (95% CI, 68%–89%) respectively, based on the study prevalence of 69% of patients with PCP. Rates of abnormal lactate dehyrogenase levels did not differ significantly between those with and without PCP.
Conclusions. Blood (1→3)-β-D-glucan is strongly correlated with HIV-related PCP. In some clinical centers, this may be a more sensitive test than the induced sputum examination and could reduce the need for both bronchoscopy and empirical therapy of PCP.
Pneumocystis jirovecii pneumonia (PCP) remains a common HIV-related opportunistic infection (OI) [1]. Because there is no available culture system for the organism, the diagnosis of PCP requires visualization of P. jirovecii cysts on microscopic examination of respiratory secretions. Noninvasive tests, such as serum lactate dehyrogenase or stains of induced sputum samples have variable sensitivity, and thus, patients may require bronchoalveolar lavage or lung biopsy for definitive diagnosis [2–4]. Novel tests with greater sensitivity, such as polymerase chain reaction (PCR) of respiratory samples, are potentially promising, but not yet widely available [5].
(1→3)-β-D-glucan (β-glucan) is a component of the wall of many fungi, including P. jirovecii. In small retrospective studies, serum levels of β-glucan have been found to be elevated in some patients who have both HIV- and non–HIV-related PCP [6–8]. We sought to evaluate the test characteristics of blood levels of β-glucan for PCP in the study population from AIDS Clinical Trials Group (ACTG) study A5164, which enrolled patients presenting with a wide range of HIV-related OIs.
METHODS
The study objectives were to outline the range of values for β-glucan in a group of HIV-infected patients presenting with diverse acute OIs, describe the test performance characteristics of β-glucan in patients with confirmed and probable PCP, and characterize the spectrum of other infectious diagnoses associated with elevated levels of β-glucan in HIV-infected patients.
The study population consisted of the 282 participants enrolled in ACTG A5164. ACTG A5164 was a strategy study of early versus deferred antiretroviral therapy in participants with acute AIDS-related OIs; the study was conducted from May 2003 through August 2007. Primary and secondary results are published elsewhere [9, 10].
Before this analysis, the diagnosis of PCP was independently adjudicated by 2 study investigators (AZ and PG) after reviewing reports from study sites. ACTG A5164 entry criteria included participants with both confirmed and probable PCP. The ACTG definition of confirmed PCP was (1) a history (within the past 3 months) of shortness of breath, dyspnea on exertion, cough, or fever; and (2) histological or cytological evidence of P. jirovecii in a bronchoalveolar lavage, lung biopsy, or sputum specimen. Probable PCP required meeting all 3 of the following criteria: (1) a history (within the past 3 months) of shortness of breath, dyspnea on exertion, cough, or fever; (2) abnormal chest radiograph (or CT scan) or hypoxemic arterial blood gas partial pressure of oxygen value <80 mm Hg or alveolar-arterial oxygen difference >15 mm Hg on room air; and (3) specific anti-pneumocystis therapy initiation. If study sites did not indicate whether PCP was probable or confirmed, the study participant was considered to have probable PCP.
Potential participants with certain HIV-related OIs were excluded from ACTG A5164 if these diagnoses were suspected or confirmed at study screening. These included tuberculosis, because of concerns about drug-drug interactions with antiretroviral drugs, and several OIs that require antiretroviral therapy for treatment (eg, progressive multifocal leukoencephalopathy, AIDS-related dementia, and cryptosporidiosis). Oropharyngeal candidiasis alone was not sufficient for entry in the study because of its generally favorable prognosis, but if present at study entry, the diagnosis was noted on case report forms. At study outset, participants receiving care in intensive care units were excluded; study enrollment criteria were modified to allow such persons to enter if they were able to provide informed consent. Participants were required to be able to take oral medications.
Stored plasma samples from each patient obtained at entry in the study were sent for β-glucan testing at Associates of Cape Cod (Fungitell Assay; Falmouth, MA). Reference values of the assay are as follows: negative, < 60 pg/mL; indeterminate, 60–79 pg/mL; and positive, ≥80 pg/mL. For calculation of sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV), β-glucan was categorized as positive (≥80 pg/mL) or negative (<80 pg/mL). Values <31 pg/mL were censored at 31 pg/mL; those > 500 pg/mL were censored at 500 pg/mL.
STATISTICAL METHODS
Comparisons of continuous and ordered categorical variables between groups were conducted with the Wilcoxon rank sum test. Categorical variables were summarized using the frequency in each category and column percentages. Comparisons of categorical variables between groups were conducted using Fisher’s exact tests.
Sensitivity, specificity, NPV, and PPV were calculated to assess test diagnostics of β-glucan for the ACTG A5164 study population. Sensitivity was defined as the percentage of persons with PCP who were correctly identified as having PCP by β-glucan level. Specificity was the percentage of persons without PCP who were correctly identified as not having PCP by β-glucan. NPV was the percentage of persons with a negative β-glucan test result who did not have PCP, whereas the PPV was the percentage of persons with a positive β-glucan test result who had PCP.
Confidence intervals (CIs) for the test diagnostics were calculated using exact binomial methods. A receiver-operator curve was presented for β-glucan as a diagnostic tool for PCP. A P value <.05 was considered to be statistically significant. Because this was an exploratory analysis secondary to the parent study A5164, results were not adjusted for multiple comparisons.
RESULTS
A total of 252 (89%) of the 282 study participants had an analyzable β-glucan result. Nine persons did not have samples available for testing, and 21 samples did not return a valid result. In 20 of these samples, the reason for uninterpretable results was optical artifact; the remaining sample was hemolyzed. The demographic and clinical characteristics of the study population are shown in Table 1. The participants had advanced HIV-related immunosuppresion, with a median CD4 lymphocyte count of 26 cells/μL (interquartile range [IQR], 10–53 cells/μL) and a plasma HIV RNA level of 5.02 log copies/mL (IQR, 4.69–5.60 log copies/μL). The most common qualifying OI was PCP (69%), followed by cryptococcal meningitis (14%) and bacterial pneumonia (9%). Participants with all forms of oropharyngeal candidiasis were combined; 44% of persons had oral or esophageal candidiasis at study entry, in addition to a qualifying OI. Participants received a median of 11 days (IQR, 9–13 d) of OI treatment before study entry.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | Value |
| Sex | |
| Male | 218 (87%) |
| Female | 34 (13%) |
| Race | |
| Black | 70 (28%) |
| Hispanic | 91 (36%) |
| White or other | 73 (29%) |
| South Africa | 18 (7%) |
| Median age (IQR) | 38 (32–44) |
| Median CD4 cell count (cells/μL) (IQR) | 26 (10–53) |
| Median HIV RNA level (log10) (IQR) | 5.02 (4.69–5.60) |
| Entry OIs | |
| PCP | 173 (69%) |
| Bacterial infection | 7 (3%) |
| Bacterial pneumonia | 23 (9%) |
| Cryptococcus | 35 (14%) |
| Mycobacterial infection | 15 (6%) |
| Toxoplasmosis | 13 (5%) |
| CMV | 9 (4%) |
| Histoplasmosisa | 8 (3%) |
| Oral/Esophageal candidiasis | 112 (44%) |
NOTE. CMV, cytomegalovirus; IQR, interquartile range; OIs, opportunistic infections; PCP, Pneumocystis jirovecii pneumonia.
Cases of histoplasmosis were enrolled in 7 of 38 participating sites.
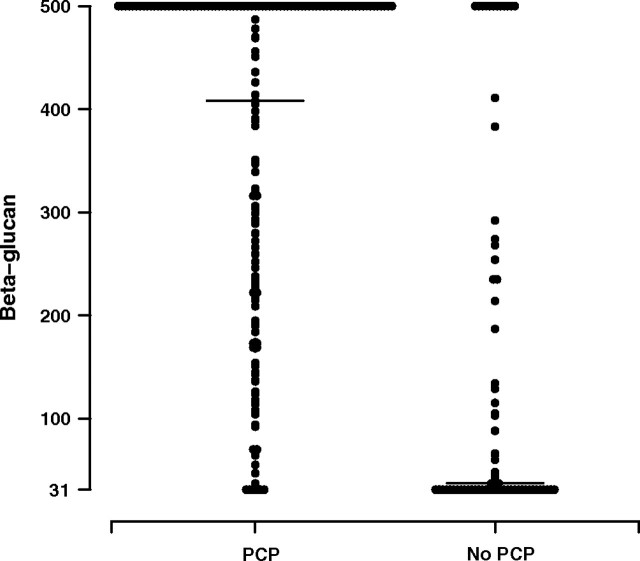
Table 2 shows β-glucan levels in participants with and without PCP. A statistically significant difference in β-glucan levels was not observed between confirmed and probable cases of PCP (P = .81), nor was a suggestion of a trend observed. The median β-glucan level in persons with PCP was 408 pg/mL (IQR, 209–500 pg/mL), with 92% of persons having a positive result (≥80 pg/mL). In persons without PCP, the median β-glucan level was 37 pg/mL (IQR, 31–235 pg/mL), with 35% having a positive result. Both continuous and ordered categorical β-glucan levels were different in persons with and without PCP (both P < .001). The results are displayed visually in Figure 1. In contrast, no statistically significant difference was seen in the proportion of participants with elevated (>400 IU/mL) levels of lactic dehydrogenase in those with and without PCP (24% vs 19%; P = .41). Levels of β-glucan did not significantly differ depending on the number of days of OI treatment before study entry. There was no statistically significant association between β-glucan levels and use of adjunctive corticosteroids (Wilcoxon rank sum, P = .70) or the composite outcome of new OI or death (Wilcoxon rank sum, P = .60).
Table 2.
Results of β-Glucan Testing by Pneumocystis jirovecii Pneumonia (PCP) Diagnosis
| PCP | ||||
| β-glucan level (pg/mL) | Yes (N = 173) | No (N = 79) | Total (N = 252) | P |
| Mean (SD) | 349.8 (163.9) | 142.0 (173.7) | 284.6 (192.6) | <.001a |
| Min, max | 31, 500 | 31, 500 | 31, 500 | |
| Median (Q1, Q3) | 408 (209–500) | 37 (31–235) | 284.5 (70–500) | |
| Negative < 60 pg/mL | 9 (5%) | 48 (61%) | 57 (23%) | <.001b |
| Indeterminate 60–79 pg/mL | 4 (2%) | 3 (4%) | 7 (3%) | |
| Positive ≥ 80 pg/mL | 160 (92%) | 28 (35%) | 188 (75%) | |
NOTE. Q1, first quartile; Q3, third quartile; SD, standard deviation.
Wilcoxon rank-sum test.
Fisher’s exact test.
Figure 1.
Distribution of β-glucan results at baseline in those with and without Pneumocystis jirovecii pneumonia (PCP). The median value for those with PCP (horizontal line) was 408 pg/mL (interquartile range [IQR], 209–500 pg/mL) and for those without PCP was 37 pg/mL (IQR, 31–235 pg/mL). Both continuous and categorical β-glucan levels were significantly different in persons with and without PCP (P < .001).
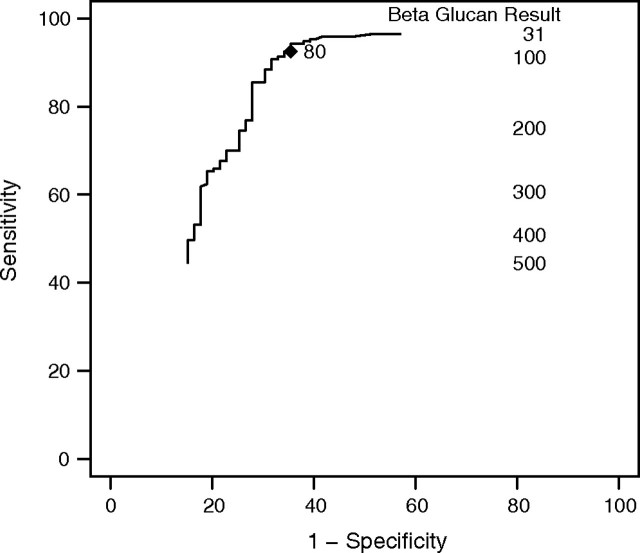
Table 3 shows β-glucan results in those with and without PCP with use of the 80 pg/mL threshold for positivity; Table 4 uses these results to calculate the sensitivity, specificity, PPV, and NPV of β-glucan for the diagnosis of PCP. The sensitivity of the test was 92% (95% CI, 87%–96%), and the specificity was 65% (95% CI, 53%–75%); for the ACTG 5164 population, this yielded a PPV of 85% (95% CI, 79%–90%) and an NPV of 80% (95% CI, 68%–89%). The receiver-operator curve for β-glucan as a diagnostic tool for PCP is shown in Figure 2.
Table 3.
Positive (≥80 pg/mL) and Negative (<80 pg/mL) β-Glucan Results by Pneumocystis jirovecii Pneumonia (PCP) Diagnosis
| PCP | |||
| Beta-glucan ≥80 | Yes | No | P |
| Negative <80 pg/mL | 13 (8%) | 51 (65%) | <.001a |
| Positive ≥80 pg/mL | 160 (92%) | 28 (35%) | |
NOTE. aFisher’s exact test.
Table 4.
Test Performance of β-Glucan in the A5164 Study Population Using ≥80 pg/mL as the Cutoff for a Positive Result
| Variable | Result (95% CI) |
| Sensitivity | 92.5% (87.5%–95.9%) |
| Specificity | 64.6% (53.0%–75.0%) |
| Positive predictive value | 85.1% (79.2%–89.9%) |
| Negative predictive value | 79.7% (67.8%–88.7%) |
NOTE. CI, confidence interval; PCP, Pneumocystis jirovecii pneumonia.
Fisher’s exact test.
Figure 2.
Receiver-operator characteristic (ROC) curve for β-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia (PCP).
β-glucan results were analyzed with respect to non-PCP infections present at study entry. The only OI besides PCP in which more than half of persons with the OI had a positive β-glucan result was oral and esophageal candidiasis (89 [79%] of 112). However, the relationship between oral and/or esophageal candidiasis and elevated β-glucan level was not statistically significant (P = .14, Fisher’s exact test), as 91 of the 112 participants with oral and/or esophageal candidiasis also had PCP. Among the 21 persons without PCP who had oral and/or esophageal candidiasis, β-glucan was positive for 5 (P = .287, Fischer’s exact test). By contrast, 6 of 8 participants without PCP who had histoplasmosis had a positive β-glucan level (P = .021, Fisher’s exact test); these cases accounted for 21% of persons without PCP who had a positive β-glucan level.
Seventy-seven (45%) of 173 study participants with PCP had levels of β-glucan >500 pg/mL, the upper limit of the assay. An additional 12 participants had a β-glucan level >500 pg/mL and did not have PCP at study entry. Three had histoplasmosis, 5 had cryptococcosis, and 1 received a diagnosis of and was treated for PCP 1 month before entering the study; 2 of the remaining participants had bacterial pneumonia (one also with oral candidiasis), and 1 additional participant had several infections (bacterial gastroenteritis, Mycobacterium avium complex infection, and oral candidiasis). Among the 13 participants with PCP who had a negative β-glucan level, 3 had confirmed PCP, 5 had probable PCP, and in the remainder, the study sites did not specify whether PCP was confirmed or probable.
DISCUSSION
In this study of participants with advanced HIV-related immunosuppresion presenting with diverse OIs, blood levels of β-glucan were strongly associated with the diagnosis of PCP. The β-glucan test had a 92% sensitivity for PCP, even though study participants had been receiving antimicrobial therapy for a median of 11 days before enrolling in the study. This sensitivity compares favorably to that reported for induced sputum examination at some clinical centers [2, 11–14].
Because β-glucan is not a species-specific marker for P. jirovecii, the specificity in this patient population of 65% for β-glucan was, not surprisingly, lower than that of stains of induced sputum (99%). Other invasive fungal infections that may trigger a positive test include candidemia, aspergillosis, fusariosis, trichosporiosis, and histoplasmosis [15–17]. Although a high proportion of study participants with oral candidiasis had a positive β-glucan level, much of this positivity was in the background of high rates of concomitant PCP among persons with thrush or esophagitis. The strong association between PCP and oral or esophageal candidiasis dates back to the early days of the HIV epidemic [18].
Our study results found that certain persons with histoplasmosis and cryptococcosis, 2 other frequently occurring HIV-related invasive fungal infections, had elevated levels of β-glucan. Indeed, in this study, elevated β-glucan levels were significantly associated with histoplasmosis (but not cryptococcosis) in those who did not have PCP. β-glucan is known to be produced by Histoplasma capsulatum, and Cryptococcus species produce it at very low levels [19]; the β-glucan test cannot reliably diagnose the latter. In our study, β-glucan level was elevated in 11 of 35 cryptococcal cases; 3 of these study participants also had PCP. Prior studies of β-glucan as a diagnostic test have also reported variable rates of positivity for individuals with cryptococcal disease. For example, Obayashi et al [20] found that β-glucan level was elevated in 5 of 6 cases of cryptococcosis; by contrast, Ostrosky-Zeichner et al [17] reported that only 2 of 12 patients with cryptococcus had a positive β-glucan level. Accordingly, the appearance of serum β-glucan positivity in the 8 of 32 persons with cryptococcus lacking PCP is similar to the rates observed by others. In cases in which clinicians might be considering both the diagnosis of PCP and either histoplasmosis or cryptococcosis, the definitive diagnosis of the latter 2 could readily be confirmed or ruled out through use of specific antigen testing [21]. Other non-PCP causes of elevated β-glucan level have been reported, most notably hemodialysis, administration of immunoglobulins, and certain antimicrobial drugs [7, 22–24].
The strong association of β-glucan with HIV-related PCP could have significant clinical implications, especially in settings where the induced sputum examination for PCP is unavailable or has a low sensitivity. Even with the availability of effective antiretroviral therapy, PCP remains one of the most common HIV-related OIs, ranking second behind only esophageal candidiasis in a recent large prospective study [1]. However, definitive diagnosis of PCP is challenging for several reasons [25]. First, the clinical presentation, physical examination, and laboratory and imaging characteristics may overlap with other processes; second, the organism cannot be cultured in clinical laboratories; and third, visualization of the organism in respiratory secretions requires special staining techniques.
Guidelines issued for management of HIV-related OIs recommend that a specific diagnosis of PCP be obtained over treating the disease presumptively [21]. However, because PCP can be difficult to diagnose definitively without broncheoalveolar lavage specimen examination, some clinicians may elect to treat empirically without confirmation. These study results suggest that β-glucan can be a helpful adjunctive test for PCP, with a negative value strongly supporting alternative diagnoses and the value of a positive result highly dependent on clinicians’ assessment of the prior probability of PCP from the clinical presentation. Indeed, since introducing β-glucan testing at our institution, we have found that β-glucan testing has increased the yield of PCP diagnoses, reduced the number of cases treated empirically, and reduced the need for bronchoscopy [7, 26, 27].
With the introduction of any new diagnostic test, it is important to consider both its relative test performance and practical issues, such as turnaround time and cost. The β-glucan assay done in this study is performed 5 days per week in a reference laboratory at a cost of $105 per test. By comparison, our institutional cost of an induced sputum examination with immunofluorescent stain for the organism is $125, not including personnel costs for obtaining the specimen. One limitation of the induced sputum procedure is that the test performance is highly operator dependent [5, 28], requiring patient preparation and cooperation, trained respiratory staff to obtain the specimen, and an experienced microbiology laboratory to visualize the organism on special stains. These characteristics likely account for the wide range of reported sensitivities for the induced sputum test published in the literature. One systematic review summarizing 7 prospective studies with paired induced sputum and broncheoalveolar lavage samples reported a sensitivity of only 55% [2]. By contrast, an early series of 51 patients (19 with PCP) cited a sensitivity of 95% with use of a highly standardized protocol [4]. It is possible that expertise in and resources allotted to diagnosing PCP by induced sputum specimens have decreased with the decrease in incidence of this OI [1].
To our knowledge, this is the largest study of a novel diagnostic test for PCP among HIV-infected individuals, with a substantially greater sample size than studies of PCR [29, 30]. An additional strength of this study is our ability to apply the findings to participants presenting with a broad range of opportunistic illnesses, not only to participants in whom the diagnosis of PCP or other respiratory illnesses are being considered [31]. Potential limitations include lack of complete information on whether PCP was confirmed or presumed and our inability to obtain a result on a subset of participants with PCP. However, we did not find a statistically significant difference in β-glucan levels between confirmed and presumed cases, suggesting that, at least in ACTG A5164 volunteers, the difference between confirmed and presumed PCP may reflect practice patterns at individual sites rather than true biologic differences. Eligible patients for the study had to be able to take oral medications, and those in whom tuberculosis was being considered as a diagnosis were excluded. These eligibility criteria could limit the generalizability of our findings.
In summary, in a group of clinical trial participants with varied HIV-related OIs, we found that plasma β-glucan level was strongly associated with the diagnosis of PCP. For settings where noninvasive testing for PCP is either insensitive or not readily available, clinicians could consider incorporating β-glucan testing in their diagnostic approach when evaluating patients with HIV infection who have respiratory infection.
Acknowledgments
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (5U01AI069472-05, for the ACTG and 1 U01 AI068634 for the Statistical and Data Management Center for the ACTG).
Beta-glucan assays were performed by Associates of Cape Cod, East Falmouth, MA. The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Potential conflicts of interest. A. Z. has received grant support from Gilead Sciences, Pfizer, BMS, VIRxSys, and G. S. K. and has served as a consultant for Gilead Sciences, Tibotec, and BMS. M. F. is an employee of Associates of Cape Cod, the manufacturer of the kits used to measure β-glucan. All other authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Buchacz K, Baker RK, Palella FJ, Jr., et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 2010; 24:1549–59. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 2.Cruciani M, Marcati P, Malena M, Bosco O, Serpelloni G, Mengoli C. Meta-analysis of diagnostic procedures for Pneumocystis carinii pneumonia in HIV-1-infected patients. Eur Respir J. 2002;20:982–9. doi: 10.1183/09031936.02.01372002. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs JA, Ng VL, Masur H, et al. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med. 1988;318:589–93. doi: 10.1056/NEJM198803103181001. [DOI] [PubMed] [Google Scholar]
- 4.Leigh TR, Parsons P, Hume C, Husain OA, Gazzard B, Collins JV. Sputum induction for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1989;2:205–6. doi: 10.1016/s0140-6736(89)90382-6. [DOI] [PubMed] [Google Scholar]
- 5.Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204–8. doi: 10.1183/09031936.03.00035303. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Nakamura T, Iwamoto A. Pneumocystis pneumonia in patients with HIV infection: clinical manifestations, laboratory findings, and radiological features. J Infect Chemother. 2007;13:1–7. doi: 10.1007/s10156-006-0484-5. [DOI] [PubMed] [Google Scholar]
- 7.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. Diagnostic performance of the (1→3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis. 2009;49:1650–9. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]
- 8.Tasaka S, Hasegawa N, Kobayashi S, et al. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest. 2007;131:1173–80. doi: 10.1378/chest.06-1467. [DOI] [PubMed] [Google Scholar]
- 9.Grant PM, Komarow L, Andersen J, et al. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PLoS One 2010; 5:e11416. doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigby TD, Margolskee D, Curtis JL, et al. The usefulness of induced sputum in the diagnosis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986;133:515–8. doi: 10.1164/arrd.1986.133.4.515. [DOI] [PubMed] [Google Scholar]
- 12.Fortun J, Navas E, Marti-Belda P, et al. Pneumocystis carinii pneumonia in HIV-infected patients: diagnostic yield of induced sputum and immunofluorescent stain with monoclonal antibodies. Eur Respir J. 1992;5:665–9. [PubMed] [Google Scholar]
- 13.Miller RF, Kocjan G, Buckland J, Holton J, Malin A, Semple SJ. Sputum induction for the diagnosis of pulmonary disease in HIV positive patients. J Infect. 1991;23:5–15. doi: 10.1016/0163-4453(91)93953-a. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Doucette S, Qian Q, Kirby JE. Yield of primary and repeat induced sputum testing for Pneumocystis jiroveci in human immunodeficiency virus-positive and -negative patients. Arch Pathol Lab Med. 2007;131:1582–4. doi: 10.5858/2007-131-1582-YOPARI. [DOI] [PubMed] [Google Scholar]
- 15.Cuetara MS, Alhambra A, Moragues MD, Gonzalez-Elorza E, Ponton J, del Palacio A. Detection of (1→3)-beta-D-glucan as an adjunct to diagnosis in a mixed population with uncommon proven invasive fungal diseases or with an unusual clinical presentation. Clin Vaccine Immunol. 2009;16:423–6. doi: 10.1128/CVI.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 17.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–9. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 18.Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study Group. N Engl J Med. 1990;322:161–5. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 19.James PG, Cherniak R, Jones RG, Stortz CA, Reiss E. Cell-wall glucans of Cryptococcus neoformans Cap 67. Carbohydr Res. 1990;198:23–38. doi: 10.1016/0008-6215(90)84273-w. [DOI] [PubMed] [Google Scholar]
- 20.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1→3)-beta-D-glucan assay for the diagnosis of invasive fungal infections–a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864–70. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207;. quiz CE1-4. [PubMed] [Google Scholar]
- 22.Kato A, Takita T, Furuhashi M, Takahashi T, Maruyama Y, Hishida A. Elevation of blood (1→3)-beta-D-glucan concentrations in hemodialysis patients. Nephron. 2001;89:15–9. doi: 10.1159/000046037. [DOI] [PubMed] [Google Scholar]
- 23.Marty FM, Lowry CM, Lempitski SJ, Kubiak DW, Finkelman MA, Baden LR. Reactivity of (1→3)-beta-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob Agents Chemother. 2006;50:3450–3. doi: 10.1128/AAC.00658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa M, Hori H, Niiguchi S, Azuma E, Komada Y. False-positive plasma (1→3)-beta-D-glucan test following immunoglobulin product replacement in an adult bone marrow recipient. Int J Hematol. 2004;80:97–8. doi: 10.1532/ijh97.04030. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301:2578–85. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 26.Marty FM, Koo S, Bryar J, Baden LR. (1->3)beta-D-glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann Intern Med. 2007;147:70–2. doi: 10.7326/0003-4819-147-1-200707030-00018. [DOI] [PubMed] [Google Scholar]
- 27.Pisculli ML, Sax PE. Use of a serum beta-glucan assay for diagnosis of HIV-related Pneumocystis jiroveci pneumonia in patients with negative microscopic examination results. Clin Infect Dis. 2008;46:1928–30. doi: 10.1086/588564. [DOI] [PubMed] [Google Scholar]
- 28.Miller RF, Semple SJ, Kocjan G. Difficulties with sputum induction for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1990;335:112. doi: 10.1016/0140-6736(90)90579-t. [DOI] [PubMed] [Google Scholar]
- 29.Huggett JF, Taylor MS, Kocjan G, et al. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jirovecii DNA in bronchoalveolar lavage fluid of HIV-infected patients. Thorax. 2008;63:154–9. doi: 10.1136/thx.2007.081687. [DOI] [PubMed] [Google Scholar]
- 30.Torres J, Goldman M, Wheat LJ, et al. Diagnosis of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected patients with polymerase chain reaction: a blinded comparison to standard methods. Clin Infect Dis. 2000;30:141–5. doi: 10.1086/313584. [DOI] [PubMed] [Google Scholar]
- 31.Desmet S, Van Wijngaerden E, Maertens J, et al. Serum (1-3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol. 2009;47:3871–4. doi: 10.1128/JCM.01756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]