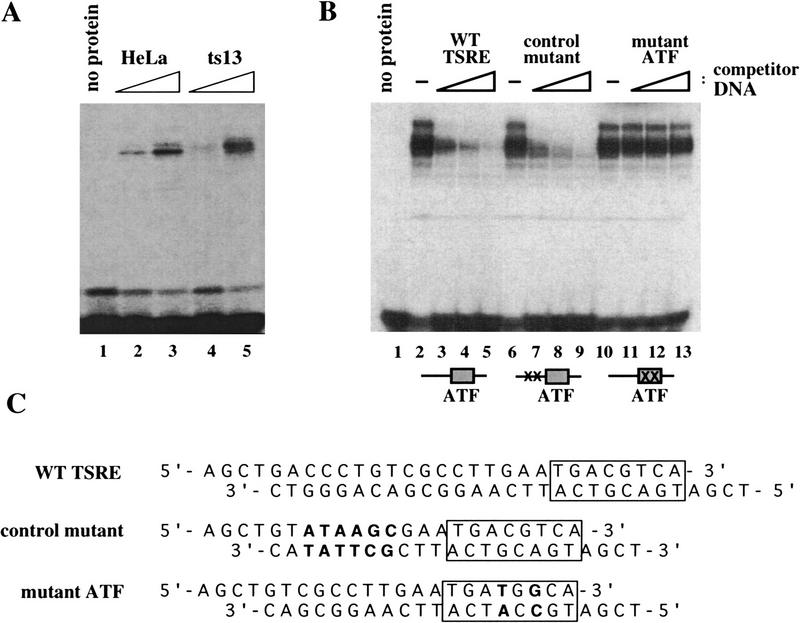
Figure 5.
Identification of cellular proteins that bind sequence specifically to the cyclin A TSRE. (A) Similar DNA–protein complexes are detected in ts13 and HeLa nuclear extracts in gel mobility shift assays. No protein (lane 1) or increasing amounts of HeLa (lanes 2,3) or ts13 (lanes 4,5) nuclear extracts were incubated with a 32P-labeled DNA probe containing the cyclin A TSRE. After 1 hr incubation on ice, DNA–protein complexes were resolved on a 5% polyacrylamide gel and detected by autoradiography. (B) Multiple proteins specifically interact with the ATF site in the cyclin A TSRE. HeLa nuclear extracts were preincubated for 15 min on ice with increasing amounts of unlabeled wild-type TSRE (WT TSRE) (lanes 2–5), control mutant TSRE (lanes 6–9), or mutant ATF TSRE (lanes 10–13) as competitor DNA followed by the addition of the labeled cyclin A TSRE DNA probe. The resulting DNA–protein complexes, after an additional 45 min incubation, were detected as described in A. A schematic representation of the competitor DNAs added to each reaction are shown below the lanes. (C) Sequence of wild-type and mutant TSRE competitor DNAs. The position of the ATF-binding site is indicated by the box. Base pair changes introduced into the mutant fragments are shown in boldface type.