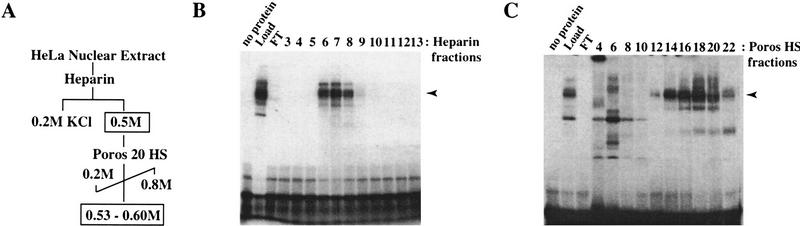
Figure 6.
Purification of TSRE-binding proteins by ion exchange affinity chromatography. (A) Fractionation scheme used to isolate cyclin A TSRE-binding proteins from HeLa nuclear extracts. (B) Protein fractions eluted from the Heparin column and containing cyclin A TSRE-binding activity as determined by gel mobility shift assays. An amount of 0.5 μl of the indicated fractions was incubated with a 32P-labeled cyclin A TSRE for 1 hr on ice. The binding reactions were subjected to 5% PAGE, and DNA–protein complexes visualized by autoradiography. The positions of DNA–protein complexes of interest are indicated by the arrowhead. (C) Elution profile of cyclin A TSRE-binding activity from Poros HS column. An aliquot (0.5–2 μl) of the indicated column fractions was assayed for TSRE-binding activity by gel mobility shift assays, as described in B. The peak of DNA-binding activity, indicated by the arrowhead, eluted between 0.53 and 0.6 m KCl.