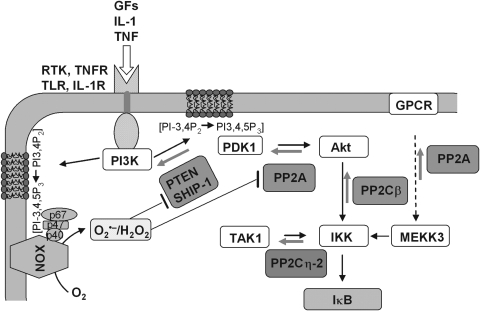
FIG. 12.
Upstream kinases and redox-sensitive phosphatases in NF-κB activation. Upon binding of stimuli to their receptors, which can be RTKs, TLRs or TNFRs, PI3-kinase is recruited to the membrane and binds to RTKs via its Src homolog-2 (SH2) domain to autophosphorylated tyrosines (72), to TLR via direct interaction with the receptor and MyD88 [reviewed in ref. (290)], and to TNFR via riboflavin kinase (525). Once localized at the membrane, the p110 catalytic subunit of PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) at the 3′ position of its inositol residue forming PIP3. PIP3 recruits Akt and phosphoinositide-dependent protein kinase-1 (PDK1), the latter phosphorylating Akt at T308 and S493 in the regulatory domain. Akt thus activated phosphorylates multiple downstream targets one of them being IKKβ. Other IKKβ phosphorylating enzymes are TAK1, activated in the canonical pathway, and mitogen-activated protein kinase (MAPK) kinase kinase-3 (MEKK3), which can be activated by G-protein-coupled receptors (GPCR). PPs counteracting the involved kinases are the dual substrate phosphatases PP2A and PP2C. PP2A has been shown to colocalize with Akt and this way inhibits PDK1 action. It also reverses MEKK3 activation. PP2Cβ reverses Akt-catalyzed IKK phosphorylation and PP2Cη-2 de-phosphorylates TAK1-phosphorylated IKK. The lipid phosphatases PTEN and Src homology-2 (SH2)-domain-containing inositide phosphatase (SHIP-1) reverse the PI3K signal by removing the phosphate groups at position 3′ (phosphatase and tensin homologue [PTEN]) or 5′ (SHIP-1). All phosphatases in the scheme can be inactivated by oxidative modification of cysteine residues. The oxidizing signal comes from NADPH oxidase (NOX) enzymes that are also activated by receptor-bound PI3K there producing the inositol phospholipid products to which phagocytic oxidase (phox) subunits can bind (107, 232).