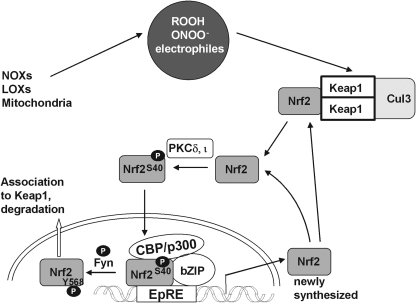
FIG. 8.
Nuclear translocation of Nrf2. Activation of Nrf2 does not necessarily require activation of a specific surface receptor. Stimuli can be exogenous or endogenously produced. In response to oxidative/electrophilic stress, the interaction of Keap1 and Nrf2 is disturbed and Nrf2 is no longer degraded. Nrf2, either released from Keap1 or newly synthesized, enters the nucleus. For translocation, Nrf2 has to be phosphorylated at Ser40. The phosphorylation is achieved by PKCδ or ι and/or other kinases (see text). Phosphorylated Nrf2 enters the nucleus, forms a complex with the basic leucine zipper (bZIP) factors CREB-binding protein (CBP)/p300, and activates gene expression by binding to the electrophile responsive element (EpRE). Phosphorylation at Tyr568 by the tyrosine kinase Fyn leads to nuclear export of Nrf2 and terminates the signal. It is not known whether Nrf2 has first to be de-phosphorylated at Ser40 or whether the bis-phosphate is exported. Exported Nrf2 can re-associate with Keap1.