Figure 7.
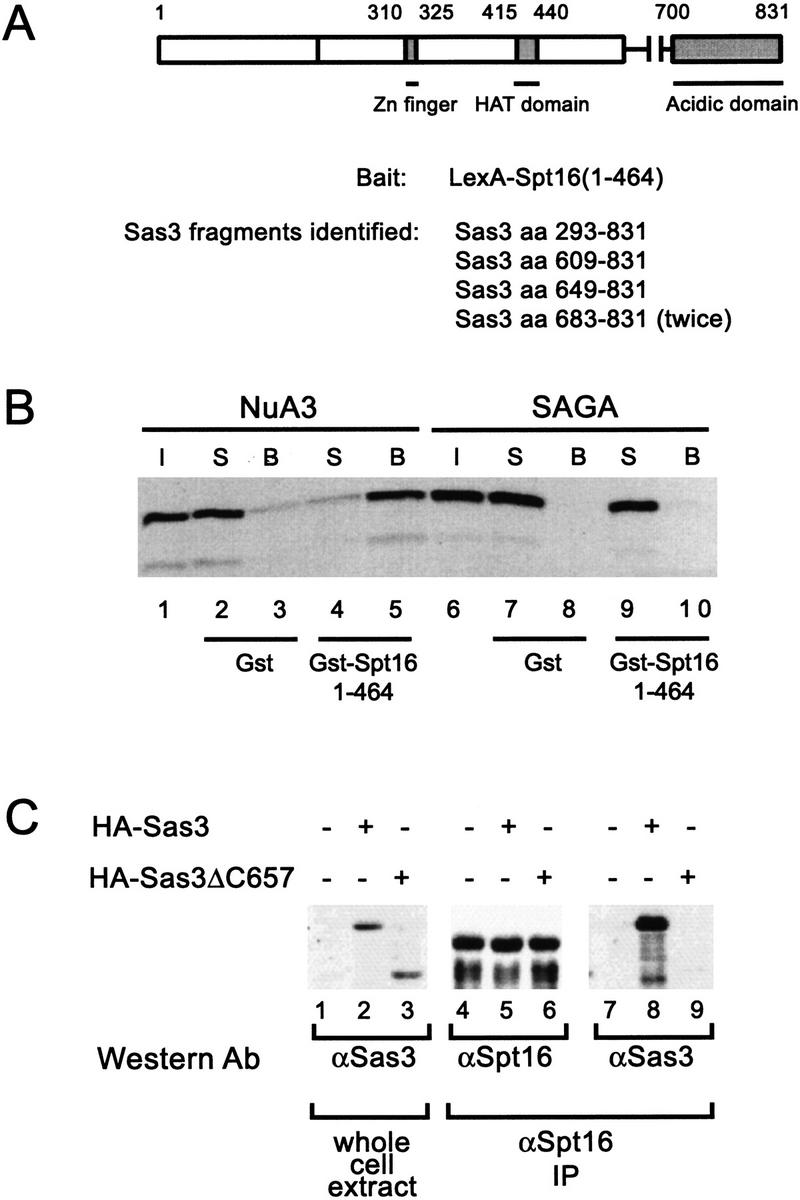
The carboxyl terminus of Sas3 interacts with Spt16, a component of a transcriptional elongation complex. In vivo and in vitro interactions between Sas3 and Spt16. (A) Schematic representation of the Sas3 protein and the Sas3 fragments identified by a two-hybrid screen using a LexA–Spt16 fusion to screen a yeast library. Sas3 fragments identified all share the common acidic carboxy-terminus. (B) Fluorogram of supernatant and beads of NuA3 or SAGA fractions incubated with either Gst or Gst–Spt16. Only NuA3 interacts with Gst–Spt16. I, Input; S, supernatant; B, beads. (C) Co-immunoprecipitation on Sas3 with Spt16. Yeast whole-cell extracts from HA-tagged Sas3 strains were incubated with anti-Spt16 antisera and precipitated with protein A–sepharose beads. Whole-cell extracts and immunoprecipitates were assayed by Western blotting with the antibodies indicated.
